 |
NYLONThe wonder material that made stockings, parachutes and toothbrushes.
Simon Cotton
Also available: HTML, Chime Enhanced and JMol versions.
|
 |
 |
NYLONThe wonder material that made stockings, parachutes and toothbrushes.
Simon Cotton
Also available: HTML, Chime Enhanced and JMol versions.
|
 |
 Why make nylon?
Why make nylon?Until the first half of the 20th century, clothing was made of natural materials - wool, silk, leather, cotton, hemp, flax... There was nothing else that could be used, and the properties of these materials were pretty fixed. At that time, structure and bonding in molecules were imperfectly understood. For one thing, it was generally believed that there was an upper limit to the size of molecules. Whilst studying the chemistry of rubber, the German chemist Hermann Staudinger (1881-1965) (photo, right) measured very high molecular masses and in 1920 he proposed the idea of small molecules joining to form very long chains, in other words, the concept of polymerisation, though this was a very unpopular opinion. By the 1930s, more and more experimental data supported this view, though it was not until 1953 that Staudinger was awarded the Nobel Prize for Chemistry "for his discoveries in the field of macromolecular chemistry".
 So who made nylon first?
So who made nylon first?In early 1928, Wallace Carothers (1896-1937) (photo, left) went from an academic career at Harvard to be in charge of organic chemistry at the new Du Pont laboratory for fundamental research at Wilmington, Delaware. His main project was to create polymers; his salary went up from $267 to $500 a month, and "as for funds, the sky is the limit". If a molecule has just the one functional group, at one end, it can only react at that end, and reaction stops there. Carothers had the idea of using monomers with a reactive functional group at each end, so each end of the monomer could react and thus form part of a chain. Despite succeeding in polymerising chloroethene and also making the first polyesters, his initial work was not commercially successful, but the research continued.
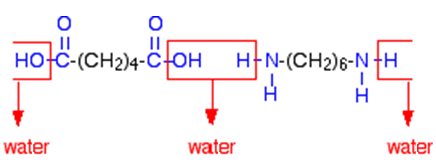
On February 28th 1935, Dr. Gerard Berchet, a member of Carothers' team, heated together hexamethylene diamine (1,6-diaminohexane, H2N-(CH2)6-NH2) with adipic acid (Hexane-1,6-dioic acid, HOOC-(CH2)4-COOH) and meta-cresol at 215°C; water distilled off, then the temperature was raised to 255-260°C to distil off the cresol in vacuo. He had made Nylon 6,6. By that autumn, the first nylon threads had been spun. With synthetic fibres, chemists could tailor substances with particular properties.
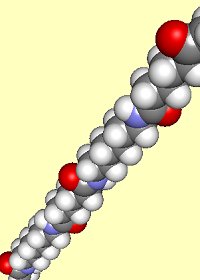
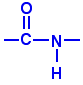 Nylon is made from two monomers, each containing six carbon atoms, so the product is known as Nylon 6,6. On reaction, an OH group is lost from the acid molecule and one hydrogen from the amine molecule, so a molecule of water is eliminated, and an amide link (see image, right) is formed between the two monomers.
Nylon is made from two monomers, each containing six carbon atoms, so the product is known as Nylon 6,6. On reaction, an OH group is lost from the acid molecule and one hydrogen from the amine molecule, so a molecule of water is eliminated, and an amide link (see image, right) is formed between the two monomers.
The loss of a small molecule in the condensation process, during the formation of Nylon and Terylene, is known as condensation polymerisation, in contrast to polymerisation of alkenes like ethane, when no atoms are gained or lost, in the process called addition polymerisation.
When this is repeated at both ends of each monomer, a chain results:
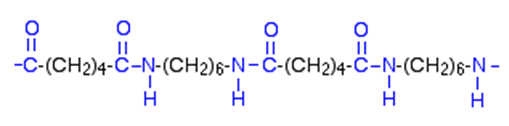
This shows two repeat units of the polymer.
Overall,
(n+1) H2N-(CH2)6-NH2 + (n+1) HOOC-(CH2)4-COOH ![]() H2N-(CH2)6-NH[CO(CH2)4-COHN-(CH2)6-NH]nOC-(CH2)4-COOH + (n+1)H2O
H2N-(CH2)6-NH[CO(CH2)4-COHN-(CH2)6-NH]nOC-(CH2)4-COOH + (n+1)H2O
Despite this success, and his election to the National Academy of Sciences, Carothers was unable to shake off the depression and mental instability that had dogged him for years. The death of his favourite sister may have been the last straw. He committed suicide by drinking potassium cyanide dissolved in lemon juice on April 29th 1937, shortly after applying for a patent on Nylon. U.S. Patent 2,130,948 'Synthetic fibers' was issued on September 20th 1938.
Another approach to making a nylon used just the one monomer, which effectively had two different functional groups, an amine at one end and a carboxylic acid at the other. "Effectively", as this molecule 6-aminohexanoic acid actually exists as a cyclic lactam (caprolactam), but this can be polymerised to form nylon-6. When this molecule polymerises, the ring opens, and the monomers link up forming a single chain of Nylon-6.
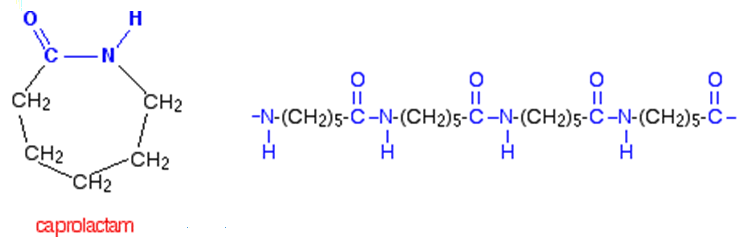
 Though investigated by the Carothers team, Nylon-6 was largely the work of Paul Schlack (photo, right) at IG Farben in Germany, who achieved this synthesis on January 29th 1938; it went under the name of Perlon.
Though investigated by the Carothers team, Nylon-6 was largely the work of Paul Schlack (photo, right) at IG Farben in Germany, who achieved this synthesis on January 29th 1938; it went under the name of Perlon.
With Nylon, Du Pont had an amazing material. Corrosion-free; easy to colour (unlike bakelite or natural materials); flexible; low density; non-biodegradeable (which we may view as a mixed blessing today). It was resistant to many chemicals, though acid tends to hydrolyse it.
The invention of nylon was made public by Charles Stine (a vice president of Du Pont) on October 27th 1938, at a meeting of womens' club members in New York; Du Pont would be targeting women for sales of the product that came to be associated with the name of their new polymer. However, the first application of nylon was in toothbrushes. Nylon bristles replaced boar's bristles, which had been hard to obtain since the Japanese invasion of Manchuria in 1937. Now Dr West's Miracle-Tuft brushes (1938) were touted as a toothbrush without bristles, which it would not shed, and which could not get soggy.

Nylon stockings weren't far behind. First offered on 24th October 1939 just to Du Pont's employees at their Wilmington, Delaware, factory, a nationwide campaign was behind their launch. May 15th 1940 was "N-Day", when nylon stockings went on sale right across America; huge queues formed and Du Pont sold 5 million pairs on that day alone, at $1.15 a pair. Expensive silk stockings quickly became a thing of the past.


 America entered World War II on December 7th 1941, and nylon rapidly became indispensable to the war effort. Parachutes were formerly made of silk, from Japan. Forthwith they were made of nylon. So were ropes, tyre cords, machinery parts like gears, life rafts...
America entered World War II on December 7th 1941, and nylon rapidly became indispensable to the war effort. Parachutes were formerly made of silk, from Japan. Forthwith they were made of nylon. So were ropes, tyre cords, machinery parts like gears, life rafts...
The switch of production to military uses meant that there was no nylon to spare for civilian uses. No nylon stockings for American women; black-market prices were astronomical. It was not until after the end of the war in the summer of 1945 that nylon was available for civilian use. Du Pont released a limited supply of stockings at just a few stores to huge queues; these stockings sold out quickly, and women rioted; the so-called "Nylon Riots". As you can see, one lady could not wait to get them home before trying them on.
It was not until spring 1946 that Du Pont was making enough for supply to catch up with demand.
|
 |
As it became an everyday material, Nylon ceased to be so novel. Increasingly, its position was usurped by polyesters, marketed under names like Dacron (US) and Terylene (UK), and celebrated in that testament to the age of plastics, 'The Man in the White Suit', played by Alec Guinness (1951). PVC came of age in the 1960s. World consumption of polymers amounted to nearly 250,000 thousand metric tons in 2006, and is increasing; much of that is still Nylon.

Poster and photo from the movie 'The Man in the White Suit'.
To make nylon 6,10, we would use 1,6-diaminohexane and sebacic acid (decane-1,10-dioic acid). In fact, this reaction uses the diacyl chloride, not the diacid, as the former is more reactive, so that the reaction can be carried out at room temperature. The sebacoyl chloride is dissolved in a hydrocarbon solvent, such as heptane (which is less toxic than the often-used hexane). The amine is dissolved in water, usually containing a little alkali. The amine solution (which is the denser of the two) is poured into a beaker or crystallising dish, then the acyl chloride solution is very carefully poured on top of it, so that the solutions do not get agitated and mix (pouring down a glass-rod is a good way of achieving this).
A nylon film forms at the interface between the solutions - use a pair of forceps and grasp the film, pulling it carefully out of the beaker, it will come as a thread. This can be wrapped round a windlass resting upon the beaker and the polymer wound up. Reaction happens where two reactants come into contact; as the nylon is removed, more molecules can meet, so nylon is formed continually as the nylon thread is wound away, and reaction continues until one of the reactants becomes exhausted. The product can be washed with water (to remove HCl etc) and left to dry.
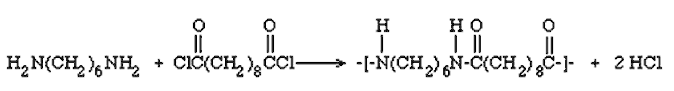
If adipoyl chloride (hexane-1, 6-dioyl chloride) is used, the product is nylon 6,6. The starting material does not keep as well as sebacoyl chloride, though.
![]()