![]()
Octanone
Attack of the Killer Mushrooms!
![]()
Paul May
Bristol University, UK
![]()
Molecule of the Month - April 2024
Also available: JSMol version.
![]()

|
OctanoneAttack of the Killer Mushrooms!
Paul May
Molecule of the Month - April 2024
|
|
You make it sound like mushrooms are predators!Actually, some are. Oyster mushrooms, for example, kill and consume nematodes (roundworms). The mushrooms produce 3-octanone in high concentrations (>50%), which is toxic to the nematodes and paralyses them. The toxin then causes their cells to die while their mitochondria become punctured and then fall apart. It sounds nasty! Are these mushrooms the only plant that uses this toxin?No, it has been identified in many different plant species, including lavender, herbs such as rosemary, basil, anise and thyme, and nectarines. This suggests that 3-octanone might be being used by the plants to protect them against being eaten by insects. But the mushrooms don’t actually go ‘hunting’ for the nematodes, do they?In a manner of speaking, yes. Oyster mushrooms (P. ostreatus) are "wood rotters" – they grow on dead trees. However, wood is relatively poor in protein, so the mushrooms grow long branching filaments (hyphae) that penetrate deep into the rotting wood in search of better protein sources. On the outer surface of the hyphae are lollipop-like structures called toxocysts which contain the toxic molecules. When nematodes encounter the toxocysts, the cysts burst, and the surrounding nematodes die within minutes. Once dead, the hyphae then dissolve the nematode remains, and absorb the nutrients. |
 An oyster mushroom (P. ostreatus) feeding on dead wood. [Image: Jörg Hempel, CC BY-SA 3.0 DE via Wikimedia Commons] |
The 3 refers to the position along the 8-carbon chain where the carboxyl group is located. This is to distinguish this isomer from the other two possible positions, 2 and 4. The actual correct name for these molecules puts the number where the ketone group is in the name, so 3-octanone should be octan-3-one.
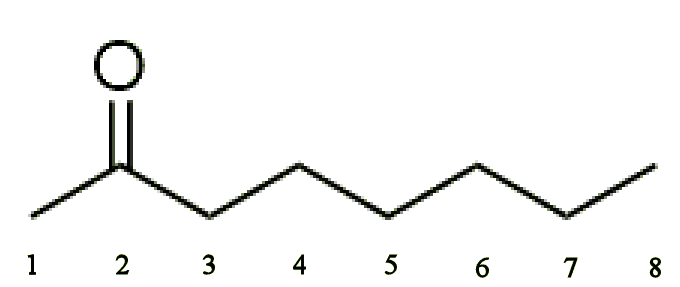 |
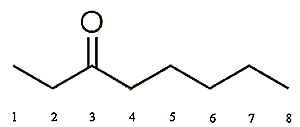 |
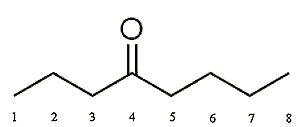 |
| 2-Octanone | 3-Octanone | 4-Octanone |
 Here, we’re only considering the n-octanones, which are the ones that have a single backbone with no side-chains. If the carboxyl group was in the 1 position (or 8 position for symmetry), then it would no longer be octanone, but octanal, as there would be a H group on one side of the C=O. Also, by symmetry, 2 & 7, 3 & 6, and 4 & 5 are identical, so we just use the lower number.
Here, we’re only considering the n-octanones, which are the ones that have a single backbone with no side-chains. If the carboxyl group was in the 1 position (or 8 position for symmetry), then it would no longer be octanone, but octanal, as there would be a H group on one side of the C=O. Also, by symmetry, 2 & 7, 3 & 6, and 4 & 5 are identical, so we just use the lower number.
Being a ketone, it has a distinctive aroma, described as sweet and earthy reminiscent of lavender. Because of this, it is used in perfumes and fragrances, and as a flavouring ingredient.
This is a colourless volatile liquid which has a very distinct odour that has been described as musty and earthy, plus the smell of blue and parmesan cheeses, sometimes described as unripe apple. This is not really surprising as it’s found naturally in butter, cheese, milk cocoa and hops. In the perfume industry 2-octanone is often used in lavender fragrances to provide a natural green-sweet-smelling effect. When ingested, the flavour is described as woody, cheesy or mushroom-like.
 |
 |
 |
| Natural sources of 2-octanone. [Images: Unsplash.com] | ||
| Butter | Cheese | Hops |
This isomer is far less commonly used than the other two. It is sometimes used as a solvent for organic synthesis reactions and can be used in the manufacture of chemical products such as paints, inks, adhesives, etc. It is also sometimes used in fragrances.
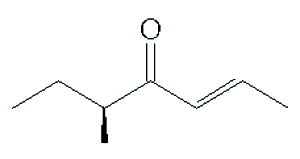
There are a lot of these – most of which have distinctive fruity aromas and so are also used in the flavour or perfume industries. One of the best known is filbertone (MOTM Sept 2012), which is the smell associated with hazelnuts.
 |
 |
Filbertone |
In fact, mushrooms make quite a bit of use of C8 molecules, not just the ketones. The MOTM from December 2009 described 1-octen-3-ol, the so called ‘mushroom alcohol’. This is a key mushroom odorant, along with 1-octen-3-one. The mushroom makes 1-octen-3-ol by enzymatic breakdown of linoleic acid, which is the most abundant fatty acid in the mushroom. Octan-3-one is regularly found too, presumably part of that biosynthetic route.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.25423513]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.25423513]