 |
1-Octen-3-ol
Simon Cotton
Also available: VRML, Chime, and VRML versions.
|
 |
1-Octen-3-ol
Simon Cotton
Also available: VRML, Chime, and VRML versions.
|
Well, some of its molecules smell of mushrooms.
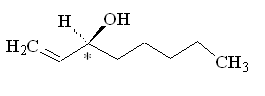
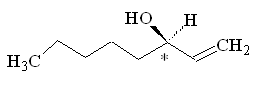
Because this molecule has a chiral carbon, with four different groups attached, it can exist as two optical isomers. One smells of mushrooms, the other smells rather mouldy.
 |
 |
(S)-(+)-1-octen-3-ol |
(R)-(-)-1-octen-3-ol |
 Why is that?
Why is that?Although the molecules have the same atoms connected in the same sequence, the atoms are oriented differently in space, so they activate a different combination of olfactory receptors. (R)-(-)-1-octen-3-ol is the main component of mushroom flavour, and is indeed known as 'mushroom alcohol' - they only produce this isomer. It is largely synthesised in the cap and gills of the mushroom.
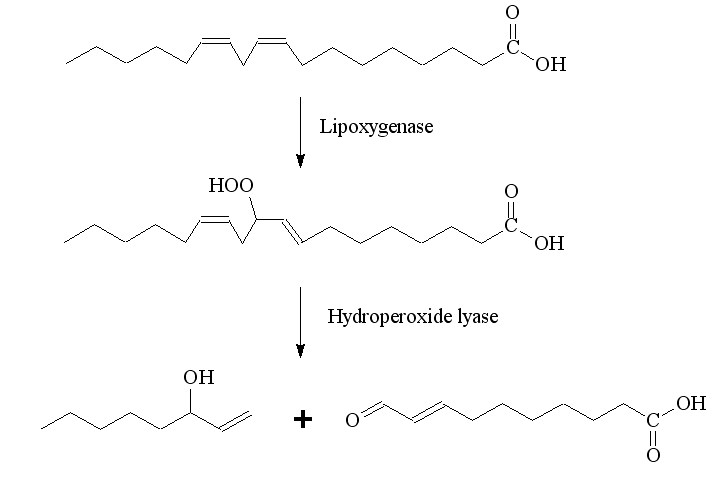
Enzymes in the mushroom break down linoleic acid, and 1-octen-3-ol is one of the products. A lipoxygenase enzyme converts linoleic acid into 10-hydroperoxy-8E,12Z-octadecenoic acid, then a hydroperoxide lyase enzyme breaks a C-C bond, generating 1-octen-3-ol and 10-oxo-trans-8-decenoic acid (ODA). In plants, different enzymes break down linoleic acid in other ways, leading to molecules like hexenal. Natural 1-octen-3-ol and ODA can be produced with a crude enzyme homogenate from mushrooms in a bioreactor, and this process is used industrially to make 1-octen-3-ol.
 So you just meet 1-octen-3-ol in mushrooms?
So you just meet 1-octen-3-ol in mushrooms?Apart from mushrooms (and truffles), it crops up in many other foodstuffs, notably giving the odour of Camembert cheese (photo, right) a mushroom note. It has also been found in blue cheeses, and in some fruit sources, such as raspberries and orange juice oil, in elder flowers and in Australian prawns and sand-lobsters.
 Some mosquitoes seem to love it. 1-octen-3-ol is in the breath and sweat of mammals, most notably cows, for whose olfactory binding protein it is the natural ligand. 1-octen-3-ol attracts mosquitoes, especially in conjunction with CO2, the two components having a synergistic effect, and has been used in mosquito traps. For this reason, there is active study of molecules such as 1-octen-3-ol which may either attract or repel insect pests, including tsetse flies (Genus Glossina) and others such as the Scottish biting midge (Culicoides impunctatus).
Some mosquitoes seem to love it. 1-octen-3-ol is in the breath and sweat of mammals, most notably cows, for whose olfactory binding protein it is the natural ligand. 1-octen-3-ol attracts mosquitoes, especially in conjunction with CO2, the two components having a synergistic effect, and has been used in mosquito traps. For this reason, there is active study of molecules such as 1-octen-3-ol which may either attract or repel insect pests, including tsetse flies (Genus Glossina) and others such as the Scottish biting midge (Culicoides impunctatus).
 Many insects like 1-octen-3-ol, and one millipede (Niponia nodulosa) (photo, right) emits 1-octen-3-ol, along with geosmin, possibly as an alarm pheromone. On the other hand, Clitopilus prunulus mushrooms are rejected by the coastal Pacific Northwest banana slug, Ariolimax columbianus, and 1-octen-3-ol is believed to be the antifeedant responsible.
Many insects like 1-octen-3-ol, and one millipede (Niponia nodulosa) (photo, right) emits 1-octen-3-ol, along with geosmin, possibly as an alarm pheromone. On the other hand, Clitopilus prunulus mushrooms are rejected by the coastal Pacific Northwest banana slug, Ariolimax columbianus, and 1-octen-3-ol is believed to be the antifeedant responsible.
One obvious route is by the selective reduction of the carbonyl group in 1-octen-3-one. Another uses a Grignard-type reaction involving acrolein (2-propenal) and amyl iodide (1-iodopentane). An elegant route starts with the teleomerisation of butadiene with acetic acid, using a palladium complex as the catalyst; this produces two products, one of these, the acetate of 1,7-octadien-3-ol, is converted to the alcohol. In a one-pot reaction, 1,7-octadien-3-ol is first converted to an alkoxide with diisobutylaluminium hydride, then LiAlH4 and Cp2TiCl2 added to selectively reduce the double bond at C7. After reduction is complete, addition of dilute HCl liberates the 1-octen-3-ol. The diisobutylaluminium group protects the hydroxy group and also masks the double bond at C1 so that it is not reduced.
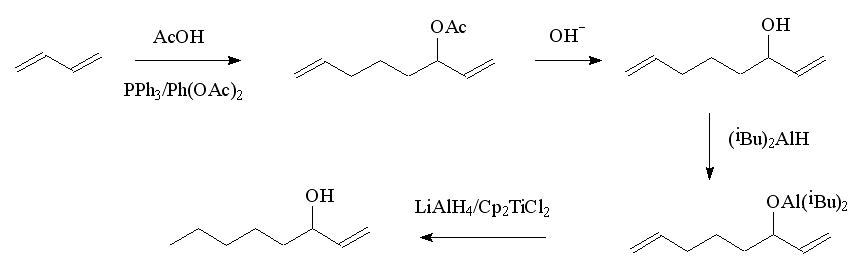
Lab synthesis of octenol.
 A future commercial source could be a member of the mint family, Melittis melissophyllum subsp. Melissophyllum (photo, right). The plant itself contains little of the compound, but on hydrodistillation of the flowering aeriel parts, the essential oil obtained was found to contain a large amount (43.6-54.2%) of 1-octen-3-ol.
A future commercial source could be a member of the mint family, Melittis melissophyllum subsp. Melissophyllum (photo, right). The plant itself contains little of the compound, but on hydrodistillation of the flowering aeriel parts, the essential oil obtained was found to contain a large amount (43.6-54.2%) of 1-octen-3-ol.
1-octen-3-ol has a very low odour threshold around 1 ppb, but a related molecule, 1-octen-3-one (structure shown below), has an even lower threshold and a very strong smell of mushrooms. It has been suggested that this oxidation product of 1-octen-3-ol contributes to the smell of mushrooms and possibly of other foodstuffs.

When a sweaty hand touches an iron object, a "musty" metallic smell can often be detected; 1-octen-3-one is the key odorant causing this, contributing about 1/3rd of the total odour concentration, along with several C6 to C10 n-alkanals and also a number of other unsaturated aldehydes and ketones. Just over 10 years ago, people were surprised by a smell of mushrooms near a Canadian car painting plant. This was traced to 1-octen-3-one formed by reaction of methanal in paint resins and methylamylketone (heptan-2-one) used as a solvent.
![]()
![]()