The Manganese-calcium oxide cluster of Photosystem II
and its assimilation by the Cyanobacteria
James D. Johnson M.S.
Alumnus, Department of Chemistry
Florida State University, Tallahassee, FL, USA
June 2006
Key: Hyperlink, Pop-up window
Introduction: This month's MOTM is an inorganic cubane complex known as the
manganese-calcium oxide cluster, commonly referred to as the "Oxygen Evolving Complex" or OEC (also referred to as a photosynthetic water oxidase). The OEC is located
on the oxidizing side of Photosystem II (PSII), and isolated within organelles known as chloroplasts,
a plastid found in all plants and algae.
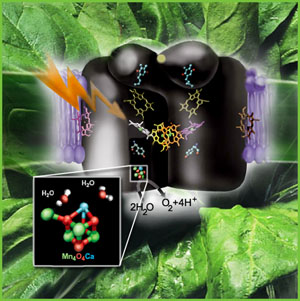 The OEC is also found in one group of bacteria, the Cyanobacteria (formally called the "blue-green algae", a name now discounted since they are in fact bacteria). It is
believed that the Cyanobacteria are the endosymbiotic ancestors of modern day chloroplasts. (1)
The OEC is also found in one group of bacteria, the Cyanobacteria (formally called the "blue-green algae", a name now discounted since they are in fact bacteria). It is
believed that the Cyanobacteria are the endosymbiotic ancestors of modern day chloroplasts. (1)
In the case of higher plants and algae, the OEC is found integrally embedded within thylakoid membranes, the latter being stacked like pancakes (grana stacks) or occur diffused within the stroma (The stroma is a term for the cytoplasmic region of the chloroplast). Photosynthetic Cyanobacteria have no internal organelles, and therefore no chloroplasts, their thylakoid membranes are simply found distributed throughout the cytoplasm or along the inner cellular wall. The Cyanobacteria are quite small, and are about the same size as the internal chloroplasts of eukaryotic plants and algae. Size is one branch of evidence that supports the theory that chloroplasts are ancient Cyanobacteria, aquired through endosymbiosis by Eukaryotic cells (additional evidence includes the occurrence of circular DNA, bacterial ribosomes, et al.). To review the components of PSII, click on the image at left to explode the details of the PSII reaction center. (Image at left from Science Beat, an on-line science newsletter published at Berkeley University (2)).
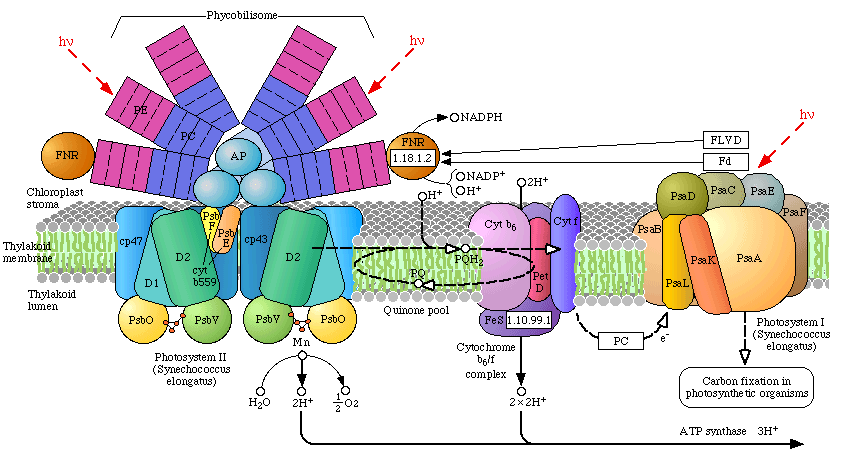
There are two types of photosystems, PSII and PSI, and it is believed that PSI is PSII's ancestor, PSII diverging from PSI early in photosynthetic evolution (more on this below). In higher plants, algae and the Cyanobacteria, both photosystems communicate with one another via an electron transport chain known as the "Z scheme" of photosynthesis. Electrons are initially extracted from water, one at a time (1 photo per electron) at the site of the OEC, and finally released to a soluble ferredoxin complex after passing through PSI (where it is further re-energized by a second photon). Ferredoxin then reduces Ferredoxin-NADP oxidoreductase, which then reduces oxidized NADP, followed by the reduction 1,3-bisphosphoglycerate. This latter reduction step regenerates Ribulose-5-phosphate followed by incorporation of CO2 by Ribulose-1,5-bisphosphate carboxylase (Rubisco) - the most abundant protein on the planet. Indeed, Rubisco is so abundant within the stroma of chloroplast, that it occurs nearly in quasi-crystalline state. The incorporation of CO2 (carbon fixation) occurs at the active site of Rubisco, part of a series of reactions known as the "dark reactions" of photosynthesis, or the Calvin Cycle (sugar production).
In algae and plants the thylakoid membranes are found partially stacked (grana) where PSII occurs in higher concentration than PSI (the stacking of the thylakoids may be related to an increase in the photon transfer efficiency of surrounding light harvesting chlorophyll complexes (LHCP) between reaction centers. Note in the pop-up window of the light harvesting complexes the concentric arrangement of the two classes of light-harvesting proteins. The inner concentric ring include the primary LHCPs (LHI), the outer rings are considered secondary light harvesting complexes (LHII). Both are under strict energetic control and may be concentrated around either Photosystem I or II. These LHCP contain primarily chlorophyll and carotenoids.
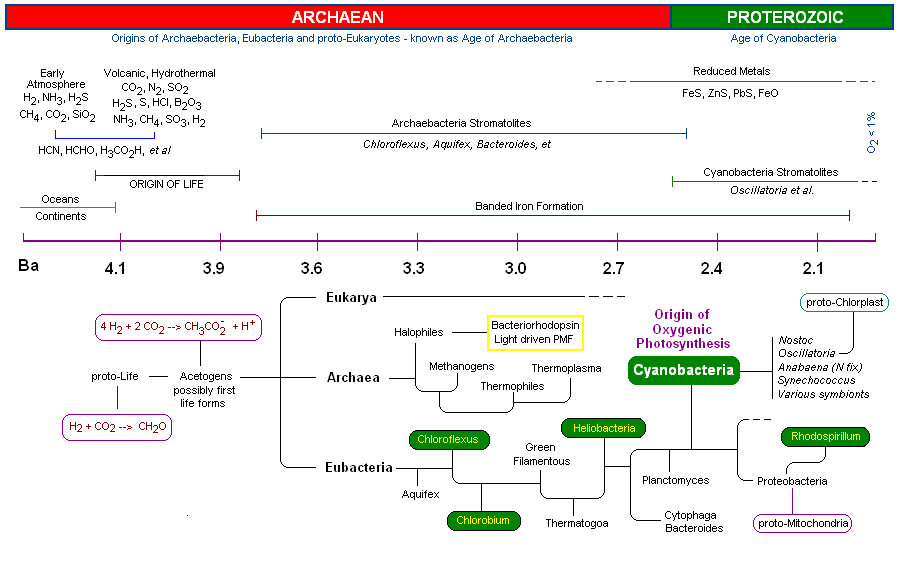
Recently the three dimensional atomic structure of the manganese-calcium oxide complex was determined by X-Ray diffraction. (3) The manganese cluster is essentially an inorganic cubane (cubical) structure found in nature as inorganic crystals of ranciéite or hollandite [Mn4CaO9*3H2O]. These minerals were most likely assimilated by early Cyanobacteria during the Archaean period, approximately 3,200 - 2,800 million years ago (or 3.2 - 2.8 Ga=giga years ago). The assimilation of the manganese-calcium oxide complex by the Cyanobacteria is one of several examples of inorganic complexes incorporated by early life for enzymatic catalytic purposes. Two other inorganic metallo complexes assimilated by early prokaryotes (and are now ubiquitous redox centers in living systems) include the cubic iron-sulfur centers [Fe4-S4] [image] (originating as greigite ([Fe4S4][SFeS]2) (4), such as ferredoxin, and the nickel-iron centers [NiFe] [image], such as Hydrogenases.
The successful assimilation of the OEC by early Cyanobacteria resulted in an phylogenetic explosion in which the Cyanobacteria became the predominant life form on earth for over 2 billion years. Unlike other photosynthetic bacteria (described below) the Cyanobacteria are "oxygenic photosynthesizers" capable of oxidizing water as a substitute for other electron donors such as H2S and reduced metals (Fe(II) e.g.).
The incorporation of a water oxidase produced molecular oxygen as a by-product, thereby setting the stage for a complete change in the earth's geologic and atmospheric chemistry, irreversibly changing the course of life on earth. During this time the Cyanobacteria successfully dispersed globally and diversified through evolutionary speciation. (5) Finally, member(s) of the Cyanobacteria symbiotically merged with eukaryotes at about 2.0 Ga to become the chloroplast of all algae and higher plant groups on the earth today. The arrival of the OEC of PSII resulted in the most profound energetic impact on the history of evolution (unlimited electron source and energy source, q.v., water and sunlight). Indeed, the OEC is had the most profound effect on, and is strictly responsible for, the rise of all higher plants and animals on the earth today.
1. First Life - the extremophilic Archaebacteria (4.0 - 3.5 Ga)
1. First Life - The Extremophilic Archaebacteria (4.0 - 3.5 Ga) Early Earth:
2. Bacterial Photosynthesis (3.5 - 2.7 Ga)
3. The Oxygen Evolving Complex (OEC) is assimilated by Cyanobacteria (2.7 - 2.5 Ga)
4. The molecular structure of the manganese-calcium oxide water splitting complex
5. A proposed mechanism for the oxidation of water by the OEC of PSII
 Fig 1. (left, top) is an illustration of the earth relatively soon after coalescing. Surface temperatures would have been very high, as well as meteorite impact frequency
(although in time the meteor impact frequency would decrease to about one impact per century by 4.0 Ga). (5) (Fig 2, left, second from
top). This early stage of the earth's history is known as the Hadean Eon, and
it was towards the end of this period that the continents were formed and the oceans accumulated (approximately 4.0 Ga).
Fig 1. (left, top) is an illustration of the earth relatively soon after coalescing. Surface temperatures would have been very high, as well as meteorite impact frequency
(although in time the meteor impact frequency would decrease to about one impact per century by 4.0 Ga). (5) (Fig 2, left, second from
top). This early stage of the earth's history is known as the Hadean Eon, and
it was towards the end of this period that the continents were formed and the oceans accumulated (approximately 4.0 Ga).
Early chaotic surface events, though becoming less frequent with time, included meteor bombardment, unstable temperatures, violent lunar forces, tides and storms, risings oceans, reduced sunlight intensity, short day length, UV, X-Ray and other high energy irradiation, volcanic and geologic upheavals and an ever changing atmospheric chemistry (6).
Earth's atmosphere at the beginning of the Hadean period was strictly reducing (lacked oxygen) (6), having measurable levels of H2, NH3CH4 and H 2S. Outgassing by volcanic and thermal vent activity released reduced metals, especially Iron, as well as Phosphorous, Sulfur, H2, H2S, CH4 and CO2 resulting in an acidic ocean. (4) Due to occasional extreme temperature conditions, exceeding 800 deg Celsius, as well as active solar flaring and other high-energy events, it is believed that CH4 and NH 3 would have been heat destroyed. So long as molecular oxygen was absent, the earth's atmosphere remained in a reduced state.
By around 4.2 Ga, the earth's atmosphere was becoming relatively stable and consisted primarily of volcanic gas. At this time the atmosphere was primarily water vapor (94%), CO (4%), N2(4%), CO, and SO 2 (7). Fig 3 (left, 2d from bottom) is an artist's rendition of the earth at about 4.0 Ga (note that the moon is much closer) corresponding to the time that Life first appeared on the earth. (8) The final Figure above, Fig 3a (bottom), represents the appearence of stromatolites, earth's first reef system occurring in shallow seas, formed by early Archaean bacteria around 3.6 - 3.8 Ga.
Hydrothermal vents and volcanic outgassing created a large reservoir of oceanic Fe(II) and FeS in the early seas. Oxidation of metals by UV radiation in oceanic surface waters may have resulted in an accumulation of Fe and Mn oxide precipitates, which subsequently settled out. (4) [see Hydrothermal vents below] These early oxidized metals, such as Fe(III), may have served as life's first electron acceptors.
First Life: The first organisms were probably extremophilic chemotropic prokaryotes (that is, tolerated
extreme environmental conditions such as high heat, high salt concentration, high and low pH, etc). The extremophiles are now known to have been early members of the
domain Archaebacteria, although it is argued that simultaneous evolution of the eubacteria as well as the eukaryotes paralleled these early extremophiles. Many species
of the archaebacteria survive today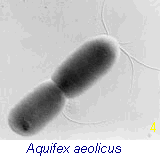 (such as the acetogens,
methanogens, thermophiles
and halophiles). These organisms, like true bacteria, lack a nucleus, as well as organized
internal membrane structures, vacuoles and internal organelles. The bacterium shown in Fig 4 (right) is genetically positioned as the oldest living fossil on the earth
today. [see Time Chart below]
(such as the acetogens,
methanogens, thermophiles
and halophiles). These organisms, like true bacteria, lack a nucleus, as well as organized
internal membrane structures, vacuoles and internal organelles. The bacterium shown in Fig 4 (right) is genetically positioned as the oldest living fossil on the earth
today. [see Time Chart below]
The early archaebacteria did however possess an amazing array of biochemical pathways to capture energy, acquire carbon, nitrogen, phosphorous, sulfur, hydrogen, bicarbonate and various organics as well as the requisite minerals needed for growth. In addition they had RNA and DNA, giving them the ability to store and pass on genetic information necessary to build enzymes to carry out the multitude of biochemical processes for life (one imagines during this time of early proto-life that modularity, symmetry and gene duplication and transfer were the order of the day (see bacterial conjugation). During the first 100 million years of life's evolution, the "tree of life" would have been more accurately described as a "web of life".
Fundamentally, the early prokaryotes had the capacity (as extant members of the Archaebacteria and Eubacteria have today) to "detect" and "respond" to changes in their environment, such as redox potentials, nutrient levels, physical events, light and temperature variances, et al. (for example, proto-7TM proteins such as Bacteriorhodopsin). The ability to respond biochemically to changes in their immediate environments allowed these organisms to adjust, tolerate, thrive, reproduce and evolve. The various hypothetical pathways and environments that led to earth's first life forms from proto-life is an area of active research. It is believed that the first organisms were acetogens or closely related methanogens [see Timeline below]. Several theories have been proposed to explain the origins of life, the most promising appears to be early hydrothermal vent systems (see below).
The oldest fossils of life on earth are found in microfossils (3.8 Ga) and stromatolites (3.5 Ga). As shown in (Fig 5, 6 and 7) stromatolites appeared in the early shallow waters of the earth approximately 3.8 Ga. The artist's rendition in Fig. 5 (below, left) of the earth at that time accurately depicts the volcanic outgassing and undersea hydrothermal vent activity that would have been prevalent near the end of the Hadean eon. The earliest stromatolites were probably pre-Photosynthetic eubacteria ancestors of Chloroflex and Chlorobium, both photosynthetic archaean anaerobic thermophiles that preceded the Cyanobacteria.

Although early sedimentary rocks have undergone extensive metamorphosis (thereby destroying microfossil remains), scientists have introduced other techniques to determine where an organism sits on the phylogenetic "tree of life". Biological macromolecules are studied for both function and sequence overlap (genetic homology, divergence, et al.) providing especially valuable insights. Indeed, this approach has become central to establishing early phylogentic relationships within the Eubacteria and Archaea. Extant biochemical pathways also lend evidence to early relationships, as well as prehistoric geochemical biomarkers, molecules and minerals left behind by species specific biological activity. Examples of this approach include the recent discovery of sterane, e.g., in 2.7 Ga sediments, that demonstrated Eukaryotes were present as well as free oxygen for biosynthesis). Radioactive analysis may also prove useful for the determination of biochemical activity.
Previous concepts of taxonomy, based primarily on morphology and physiology, have been supplanted by the work of Woese et al. who revolutionized the technique of using DNA as molecular fossils (10). Woese and his group are responsible for the discovery of the domain Archaea, and in the process laid the groundwork for future research in this area.
Geochemists who study early life suggest that life may have originated in and around undersea hydrothermal vents (see Michael Russell's First Life, referenced below). Hydrothermal sites are excellent locations ideally suited for first life for a number of reasons. Undersea thermal vents provide protection from UV radiation, which would have restricted life from establishing itself on the surface (whether marine, aquatic or terrestrial). Hydrothermal vents would also have provided long-term stability with regards to a number of parameters, such as pH, temperature, organic nutrients and inorganic minerals.
As pointed out by Dr. Russell and Dr. Hall (4), hydrothermal vents provide a warm, constant temperature springs (hydrothermal vents have recently been discovered with temperatures near 90 deg C rather than the typical "black smoker" temperature of 400 deg C. - link is to video of the "Lost City" thermal vents in the mid Atlantic). Thermal stability maintained over long periods of time (millions of years) would ideally accommodate the environmental needs of early life. The early Hadean oceans are believed to have been acidic and the effluent emanating from thermal vents are known to be alkaline (perhaps the first open door to a protomotive force, maintaining a constant gradient which early life may have taken advantage of). In the Jan 2006 edition of the journal American Scientist, Dr. Michael Russell presents several models of early life processess in and around these thermal vent systems.
One of the most attractive ideas of thermal vents as candidates for nursing first life is their geochemistry. Early in the history of the earth reduced
minerals such as Fe(II) and Ni(II) would have steadily been released by these systems. As reduced metals entered into
 the acidic environment of the sea, the change is temperature may have facilitated precipitation as carbonates, silicas, clays and iron-nickel sulfides. These
precipitates, like stalagmites in a limestone cave, would have accumulated and built up around the vents (which is evident in these systems today). It is known that these
structures are also cavitous and porous, thereby providing ideal breeding grounds for the compartmentalization of first life.
the acidic environment of the sea, the change is temperature may have facilitated precipitation as carbonates, silicas, clays and iron-nickel sulfides. These
precipitates, like stalagmites in a limestone cave, would have accumulated and built up around the vents (which is evident in these systems today). It is known that these
structures are also cavitous and porous, thereby providing ideal breeding grounds for the compartmentalization of first life.
One could imagine as well, as has been proposed by Russell et al., that many of these vents were in, or otherwise found their way to (by crustal plate techtonics) fairly shallow water where further oxidation and precipitation of effluent minerals may have been facilitated by surface UV radiation. Hydrothermal vents may have been life's first "flow reactor" (as characterized by Dr. Russell); providing a long term stable environment, with plenty of nutrients and minerals, as well as thermal and chemical potential that early proto-life may very well have taken advantage of.
Fig 8, left, shows a typical black smoker hydrothermal vent. This particular vent may be one of the higher temperature vents (400+ deg Celsius), but perhaps cooler vents, in the vicinity of 100 deg Celsius, may have provided opportunity for early thermophilic prokaryotes to establish a foothold. Until recently no fossilized evidence of early prokaryotes had been reported around hydrothermal vents (at least prior to 544 Mya). Fig 9, left, shows an image of a proposed early prokaryote (pyritic filaments) found in a 3.235 Ga volcanic sulphide deposit from the Pilbara Craton of Australia. (9) Dr. Birger Rasmussen, the scientist from the University of Western Australia who reported this find, proposed that this was evidence of a thermophilic chemotropic prokaryote, embedded within a matrix of pyrite (more than likely an iron-oxidizing prokaryote), and inhabited a sub-sea-floor hydrothermal environment. This finding would be in line with the proposal that life originated at the base of ancient hydrothermal vents. Though this finding is not absolute proof standing alone (some controversial results were seen in the literature regarding this find) it does represent the efforts that are underway to continue looking for signs of early life around ancient fossilized venting systems. It is important to note that the OEC would have required the presence of manganese, especially in the form of the cuboidal manganese oxide clusters, as described above, and that thermal vents are known to be rich in these minerals.
The appearance of alternate layers of oxidized and reduced iron deposits known as the ‘banded iron formations’ were initially argued to be evidence of early oxygen
evolution, oxygen being responsible for the periodic deposition of iron oxides (dark red in image at right).
 conditions. Banded iron deposits appears in the geologic record very early, around 4.0 Ga, which is now believed to be too early for the rise of the Cyanobacteria, which would
have been responsible for early oxygen release. It is believed that these early oxidized/reduced iron bands were probably generated by chemolithoautotrophic iron oxidation or
by photoferrotrophic prokaryotes in a oxygen free environment (16) or, alternatively, they may be completely abiogenic in origin (19).
conditions. Banded iron deposits appears in the geologic record very early, around 4.0 Ga, which is now believed to be too early for the rise of the Cyanobacteria, which would
have been responsible for early oxygen release. It is believed that these early oxidized/reduced iron bands were probably generated by chemolithoautotrophic iron oxidation or
by photoferrotrophic prokaryotes in a oxygen free environment (16) or, alternatively, they may be completely abiogenic in origin (19).
By 2.5 Ga, oxygenic photosynthesis was beginning to make a clear mark in geologic sediments. Cyanobacteria, by assimilating the OEC and releasing molecular oxygen, changed the geological landscape of the earth, as well as its atmosphere and oceans. All other organism would respond in abeyance, either taking up oxygen tolerant metabolism or recess into niches devoid of oxygen. The assimilation of the manganese-calcium complex by the cyanobacteria laid the groundwork for the Cambrian explosion, and for the evolution of the animal and plant kingdoms that followed.
2. Bacterial Photosynthesis (3.8 - 2.7 Ga)
Introduction to the Photosynthetic bacteria: Thirty-six bacterial lineages (Eubacteria) have been identified, of which only five are capable of using chlorophyll-based energy conversion to create a Protonmotive Force (PMF) to drive ATP synthesis and reduce CO2 to sugars. Of the five bacterial groups capable of photoautotrophic photosynthetic growth, four, (the exception being the Cyanobacteria), perform photosynthesis under anaerobic conditions and do not oxidized water to molecular oxygen via the OEC (anoxygenic photosynthesis). Indeed, the Cyanobacteria are the only group of bacteria that have incorporated the OEC necessary for splitting water as a source of electrons. The majority of the Cyanobacteria are obligate photolithoautotrophs, having very limited capacity to photoassimilate organic compounds. The ability to photosynthesize and fix Nitrogen as resulted in many members of the Cyanobacteria to become endosymbionts with plants, lower animals and fungi (lichens).
Members of the five photosynthetic bacterial families are shown below (these are example species from each group):
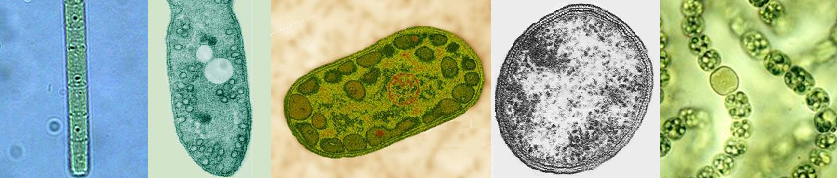
|
| From l to r: Chloroflexus (green non-sulfur bacteria, Chloroflexaceae), Rhodospirillum (purple bacteria, Rhodospirillaceae), Chlorobium (green sulfur bacteria, Chlorobiaceae), Heliobacterium (Gram-positive, Heliobacteriaceae), and Nostoc (Cyanobacteria, Nostocaceae). Note the N2 fixing heterocyst in Nostoc. |
Table 1. A tabular summary of of the various physiological parameters of photosynthetic bacteria
|
Major Groups of Photosynthetic Phototrophic Prokaryotes |
|||||
| Type | Bacterial Group | Pigment(s) | Primary Products | e- donors | Carbon Source |
| Anoxygenic | Halobacteria | Bacteriorhodopsin | ATP | None | Organic |
| Anoxygenic | Filamentous Green | Bchl a | ATP | Organic, S2–,S2O32– | Organic, CO2 |
| Anoxygenic | Green Sulfur | Bchl a | ATP + NADH | H2, S2–, S0,S2O32– | CO2 |
| Anoxygenic | Purple | Bchl a or b | ATP | H2, S2–, S0,S2O32–,organic | CO2 and/or organic |
| Anoxygenic | Heliobacteria | Bchl g | ATP + NADH | Organic | Organic |
| Oxygenic | Cyanobacteria | Chl a | ATP + NADH | H2O | CO2 |
| Photosynthesis in Eubacterial Prokaryotes: Mechanisms and Machinery | |||||
One of the most fascinating areas of biochemistry today is the determination of evolutionary relationships among early prokaryotes (including all three domains, see time line below). Recent findings have revealed that early prokaryotes are highly heterophyletic. Scientists now believe that the transfer of genetic information between divergent organisms has been prolific, especially very early in evolution, thereby complicating further the genomic mapping of their ancestry (genes may be transferred between divergent organisms though such processes such as conjugation, as previously discussed. In the case of the Prokaryotes, transformational have events literally turned the "tree of life" into a three dimensional "web of life." With species dying out in the interim, and evolution spanning more then 3,000 million years, bio- and geo-chemical researchers have plenty to keep them busy for the foreseeable future. A time line was compiled while reading the various articles to write this MOTM page and is shown below (Best estimates are plotted. The diagram has many hyperlinks embedded within it (identified by the appearence of a hand when the cursor is moved over a hyperlink)).
Before leaving the Eubacteria and taking a closer look at the photosynthetic apparatus of the OEC, an academic class exercise designed to take a look at the Eubacteria and their lifestyles is worth mentioning. An excellent exercise for teachers to perform in a biology class is to set up what is known as a "Winogradsky Column". The column is designed to demonstrate the interdependence of various bacteria on metabolites and metabolic wastes. Using pond material a large graduated cylinder is filled and allowed to settle for 1-2 months. Within a few weeks three distinct layers begin to separate out, q.v., (1) aerobic water, (2) anaerobic water, and (3) anaerobic sediment. Anaerobic organisms occupy the oxygen free zones and the Cyanobacteria the aerobic zones. The procedure for setting up this exercise can be found at Dr. Jim Deacon's Molecular Biology demonstration site at the University of Edinburgh.
Bacterial reaction center and light harvesting chlorophylls:
All known microorganisms use two functionally unrelated processes for the conversion of light into chemical energy. Chlorophyll-based systems are used by the photosynthetic bacteria to drive photosynthesis. Light harvesting chlorophylls complexes, phycobilisomes and chlorosomes make up the principal systems of light absorption by these organisms. All utilize one of two basic types of photosynthetic reactions centers (known as RC1 and RC2, see below). Halobacterium salinarum, a Halophile, uses bacteriorhodopsin (bR) to convert light into a proton gradient (primarily used to maintain salt balance in hypersaline environments and not ATP formation). The active component is a trans-membrane protein complex known as Bacteriorhodopsin (bR). bR is considered one of the 7TM sensory peptides (arising by gene duplication events) which occurs across a wide range of plants and animals and are utilized for various sensory adaptations. Note too that the various photosynthetic electron transport schemes are fundamentally different between the Cyanobacteria, the Purple bacteria and the Green Sulfur bacteria (pop are added for a quick overview but non-oxygenic photosynthesis is not discussed here).
Of the photosynthetic bacteria, three schemes are used for light harvesting pigments. Chlorobium spp. uses a specialized internal membrane bound body known as a Chlorosome (see below). Chloroflexus spp., Heliobacteria spp., and Rhodospirillum spp. use a class of Light Harvesting proteins (below right) in which bacteriochlorophyll is concentreically embedded and arranged around a reaction center to focus and transfer light to the center (below left). Finally, the only Eubacteria to use the OEC in oxygenic photosynthesis utilize Phycobilisomes (see below). Throughout the Eubacteria there are various arrangements of these light harvesting pigments in their association with the photosynthetic reaction centers. All of the photosynthetic pigments are designed to absorb visible light across a range of wavelengths suitable for any number of environments.
Light Harvesting Chlorophyll: A typical bacterial reaction center is shown below, left. Note the bacteriochlorophyll reaction center "dimer", a special pair of chlorophyll molecules (yellow with green Mg++) designed to absorb incoming photons from surrounding light harvesting chlorophyll complexes. The light blue Mg++ free bacteriochlorophylls are special chlorophyll molecules known as Pheophytins. Pheophytins transfer electrons from the reaction center dimer to a pair of quinones (shown in red). The solitary orange atom represents iron which serves to stabilize conformation and transfering electrons between quinones.
Surrounding the bacterial reaction center in several bacteria species, algae and plants are complexes known as Light Harvesting proteins (below right). These proteins bind chlorophyll (in this case bacteriochlorophyll) molecules and other pigments such as lycopenes and carotenoids (note that one role carotenoids play is to prevent the reaction center from becoming photo damaged by over oxidation). The light transferring efficiency of protein-chlorophyll complexes from initial absorption until the photon reaches the reaction center is amazing. The chlorophyll molecules that are held within the protein complex (green molecules in image below right) are positioned to maximize photon transfer. The electron path after reaching the dimer moves through the pheophytins (light blue) and on to the quinones (red, below image left). The reaction center dimer is then reduced by an external electron sources such as Cytochrome or, in the case of the Cyanobacteria, algae and plants, the oxygen evolving complex (OEC). A brief review of the various light harvesting arrangements is given below. The PSII complex is much more sophisticated then the simple bacterial light harvesting complex shown below.
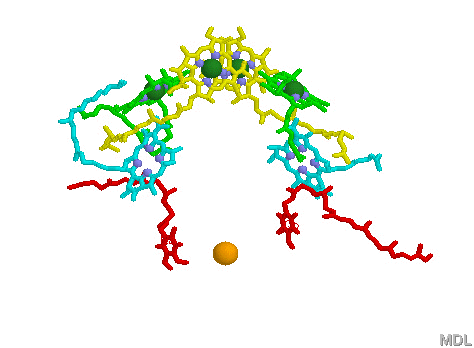
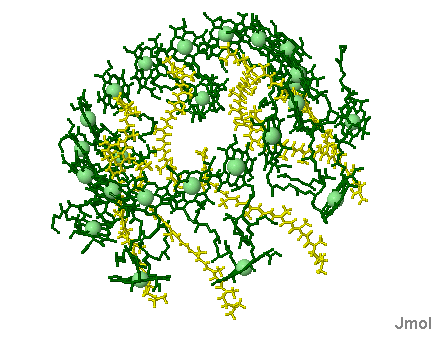
Phycobilisomes: Phycobilisomes are light harvesting pigments found throughout the Cyanobacteria (and in red algae). All other photosynthetic bacteria use the light harvesting complex as described above. Phycobilisomes, which specialize in absorbing deep penetrating green light (more than 1 meter deep, e.g., in marine environments), are capable of re-emitting light in regions which the other photosynthetic pigments can absorb. This would be a very advantageous adaptation in marine environments in which protection from UV radiation might be needed. There is a central core of allophycocyanin (AP) which sits above the photosynthetic reaction center. There are phycocyanin and phycoerythrin subunits which radiate out from this center like thin tubes. The fluorescent pigments which are present in the phycobilisome, such as phycocyanobilin (PC) and phycoerythrobilin (PE) re-emit the green light in regions.
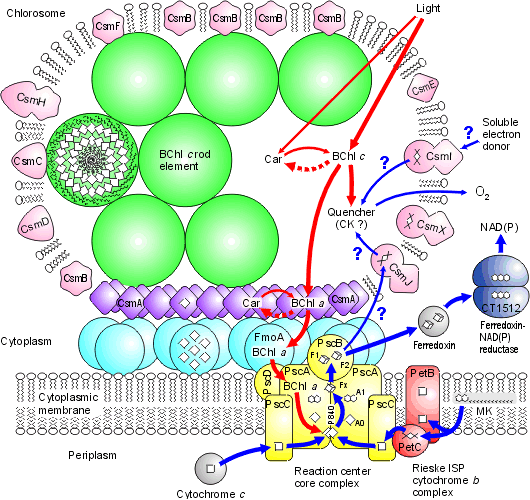
Chlorosomes: Model of the photosynthetic apparatus in Chlorobium tepidum showing "chlorosome" body compiled by Dr. Donald Bryant and associates at Penn State University (Chlorosomes found in the green sulfur bacteria, family Chlorobiaceae). Light and excitation energy transfer is shown in red, electron transfer is shown in blue. Proteins and chlorosome rod elements are shown in color and some cofactors are indicated as white symbols. Diamonds represent individual BChl c molecules in the chlorosomal rod elements, BChl a molecules in CsmA, FMO protein, and reaction center (the double diamond represents the P840 special pair), and the primary acceptor Chl aPD in the reaction center; cubes represent [4Fe-4S] clusters in the reaction center and soluble ferredoxin; rhomboids represent [2Fe-2S] clusters in CsmI, CsmJ, CsmX, and PetC; squares represent heme groups in the reaction center, PetB, and soluble cytochrome c; double hexagons represent menaquinone molecules in the reaction center and cytoplasmic membrane; triple hexagons represent a FAD cofactor.
3. The Oxygen Evolving Complex (OEC) is assimilated by Cyanobacteria (2.7 - 2.5 Ga)
The evolution of two Photosystems: The evolution of the oxygenic photosynthetic reaction center is has become a leading area of evolutionary biology. Evolution has produced two photosystems, known as Reaction Center Type I (RC1 or PSI) and Reaction Center Type II (RC II or PSII). The OEC operates on the oxidizing side of RC II, but we find that both Photosystems are used by prokaryotes with slightly differing functions and evolutionary adaptations. Cyanobacteria use the oxygenic PSII and PSI electron transport system as does algae and plants. Anoxygenic bacteria use either RC I, or a prototype of RC II, depending on species and environment. At least one organism, Oscillatoria, has both.
To understand the various distribution and functions of the two reaction centers, and how they might be related evolutionarily, John F. Allen (Dept of Biology, Queen Mary, University of London) recently published a model to explain the appearance of the two reaction centers throughout the prokaryotes and higher plants and algae. It is now generally accepted that PSII evolved from a primitive PSI, the two reaction centers are in fact homologous in both function and structure (determined from kinetic, X-Ray and genetic studies). Dr. Allen calls his model a "redox switch hypothesis" in which the redox levels in the environment (amount of available reductants, etc) may trigger a bacterium to switch from one reaction center to another. The associated light harvesting chlorophyll proteins, being integral within the bilipid membrane, are capable of serving as light harvesting complexes for both photosystems. Dr. Allen's model is presented below.
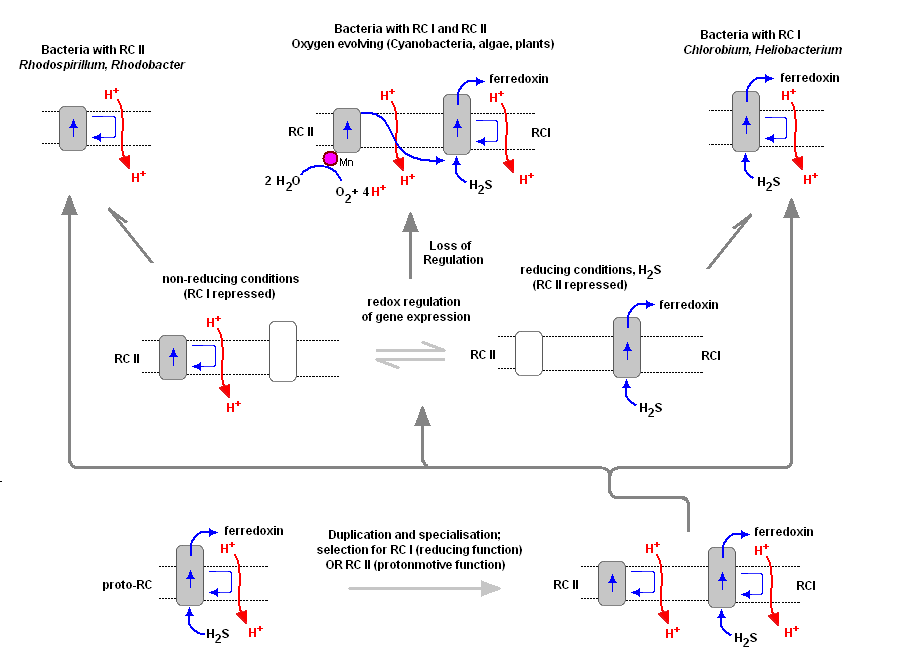
The original RC I is shown in the lower left, labeled as proto-RC. This reaction center operates with external reductants, such as hydrogen sulfide, and is capable of supplying electrons to a quinone pool, which can then recycle the energized electrons back to the reaction center while moving protons across the membrane into the periplasmic space thereby creating a protomotive force for the synthesis of ATP. In addition, the RC I provided electrons to soluble electron acceptors (ferredoxin) for use in organic synthesis.
Over time and changing conditions, a second independent photosynthetic reaction center emerged, under a scheme of genetic control that would favor one over the other. Allen argues that in this early stage the two reaction centers would have been cooperative, their expression genetically controlled by changing environmental conditions (alternatively, there may have been initial competition). The rise of the RC II enabled the bacteria, under conditions in which a reductant, such as H2S, was readily available to support photon-driven proton pumping through the quinone cycle into the periplasmic space for ATP synthesis. Single reaction center anaerobic phototropohs were either photolithotrophic (RC 1) or photoorganotrophic (RC II). Dr. Allen argues that metabolic flexibility may have selected those bacterial capable of providing one or both of the two types of photosystems, depending on environmental conditions.
Over time bacteria appeared (such as in the present day Oscillatoria) in which both photosystems are present, and are synthesized and distributed through the membrane depending on environmental conditions encountered (Allen proposes this is carried out under redox control of gene expression). Oscillatoria limnetica has both RC I and RC II, and under conditions of low H2S, switches to RC II and performs oxygenic photosynthesis (Oscillatoria is a member of the Cyanobacteria. Note that there are other conditions, such as the heterocyst of Nostoc, in which RC II is shut off, as oxygen will interfere and shut down nitrogen fixation. See also C4 cell et al.).
As the image above shows, bacteria with RC II only are known (anoxygenic), as well as bacteria with RC I only (also anoxygenic) Dr. Allen proposes that at some point in time the genetic "redox switch" was not longer needed and the two photosystem began to specialize and work in cooperation. Genetic regulatory control can still take place by either lowering or increasing either the reaction centers themselves or distributing their associated light harvesting complexes between the two.
4. The molecular structure of the manganese-calcium oxide water splitting complex
Resolution: The recent 3.5 Angstrom resolution of the OEC has opened a black box that has been closed for many years. Earlier kinetic and spectroscopic data are falling in line with the mechanism of this complex but the race is on to determine exactly how the oxygen evolving complex extracts electrons from water. We now know there are 4 manganese atoms (their respective redox states and behavior has not been determined with certainty), a calcium atom, now believed to be an essential component of the water oxidizing site, and a chloride (Cl-) co-factor. Chloride does not appear to be essential and is believed to play a charge stabilizing role during water oxidation. An excellent animation of the Photosystem II complex, including the light harvesting chlorophylls, the reaction center and the OEC can be downloaded at Dr. Johannes Messinger's site (36 megs, Windows, RealTime) and is a beautiful rendition of PSII (highly recommended).
Dr. Messinger suggests that once the mechanism of water oxidation has been determined, it may be used to engineer a renewable man-made energy source by combining the oxidation of water with a hydrogenase (future solar hydrogen and oxygen production).
The mechanism of water oxidation has eluded scientists for decades. In the last 50 years much work has been carried out on the kinetics of PSII (OEC). Electrophoresis and related techniques carried out over the last 30 years had identified most of the polypeptides associated with PSII. New X-Ray diffraction studies have identified the stereochemistry and atomic structure of the OEC and surrounding complexes, though there is still debate over the number of ligands involved, their types, and how many of the ligand positions may be occupied by water. Once the mechanism of water oxidation is elucidated, it will celebrated as one of bilogy's greatest discoveries since the discovery of DNA's 3D structure in 1954.
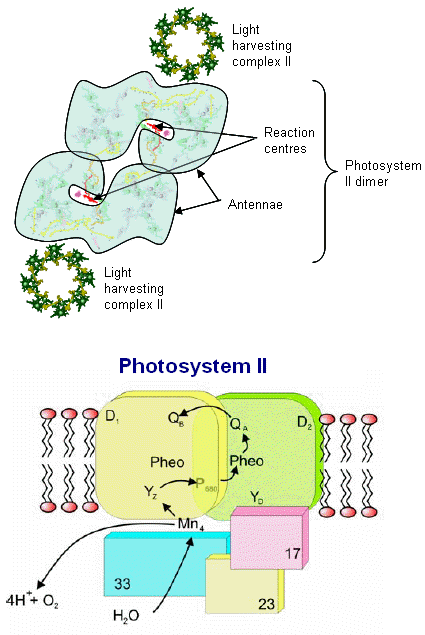 Photosystem II consists of an array of proteins, held together by numerous salt links, hydrogen bonds, water molecules et al. and can become especially
labile under stressful conditions such as high temperatures and low pH. The complex is also sensitive to photoinactivation if electron flow throw the "Z"
scheme is not delicately balanced (ATP phosphorylation of the surrounding light harvesting chlorophyll proteins is part of this regulatory scheme.
Photosystem II consists of an array of proteins, held together by numerous salt links, hydrogen bonds, water molecules et al. and can become especially
labile under stressful conditions such as high temperatures and low pH. The complex is also sensitive to photoinactivation if electron flow throw the "Z"
scheme is not delicately balanced (ATP phosphorylation of the surrounding light harvesting chlorophyll proteins is part of this regulatory scheme.
From the image at left, PSII is diagrammatically depicted as a dimer, as it is found embedded in the thylakoid membranes (see Dr. Messinger's video, which clearly shows the central axis of this complex). The PSII reaction center complex is surrounded by additional light harvesting chlorophylls, shown here as LHCII (only 2 are shown, these would completely surround the complex in a natural state.
The various proteins that surround the PSII complex are shown in the diagram at left. They include the principal Light Harvesting Complexes (LHCI), D1, and D2, as well as associated peptides including the 33 kdal, 23 kdal and 17 kdal. These periphery proteins support the reaction center and associated cytochromes and other pigments.
The general reaction is also outline (detailed below) in which the reaction center chlorophyll dimer, P680, is initially oxidized by an incoming photon, the
electron being passed to the nearby pheophytin and then transferred to the quinone pool. Manganese, bound to water, calcium, chloride and oxygen (in manganese oxo bridges) then rapidly
reduces Yz (a local Tyrosine) which itself became oxidized when it reduced the oxidized P680+. 35 years ago Joliot and Kok (see Photosynthesis references below) worked out the
mechanics of the four step cyclic process required for turning over PSII during its water oxidation event. Each PSII reaction center within the membrane works independently of
one another and cycles through a set of 5 oxidation states, known as the "S" states (So![]() S4). Our current understanding
of the mechanism of water oxidation proceeds through these four states known as the "Kok Scheme" (for more details on the structure of PSII proper see the references
and links provided below.
S4). Our current understanding
of the mechanism of water oxidation proceeds through these four states known as the "Kok Scheme" (for more details on the structure of PSII proper see the references
and links provided below.
|
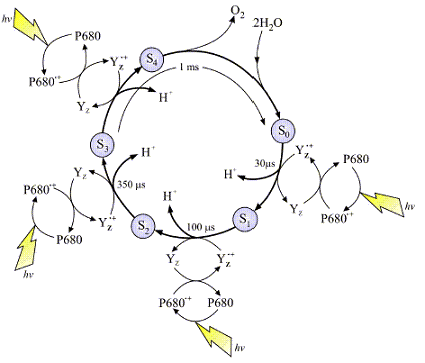
The image at left is a typical representation of the Joliot-Kok Cycle. This particular schematic is from an article published by
Dr. James Barber, Imperial College of London, International Journal of Photoenergy 6:43-51 (2004).
(Take the time to visit Dr. Barber's web site to see his Photosystem II images.
The 4-step Kok Cycle is represented schematically in the image at left. Four incoming photons are required to complete the process. The OEC starts at State So, and
then with each incoming photon moves on to Sate S1, then S2, S3 and finally S4. There are a total of five oxidation states of the OEC during this process. As the OEC cycles through
this process four electrons are removed from bound water (see mechanism below). The deposition of protons during this process varies but it is believed 2 protons are released during
the final S3 |
The X-Ray data and model of the OEC are shown below.
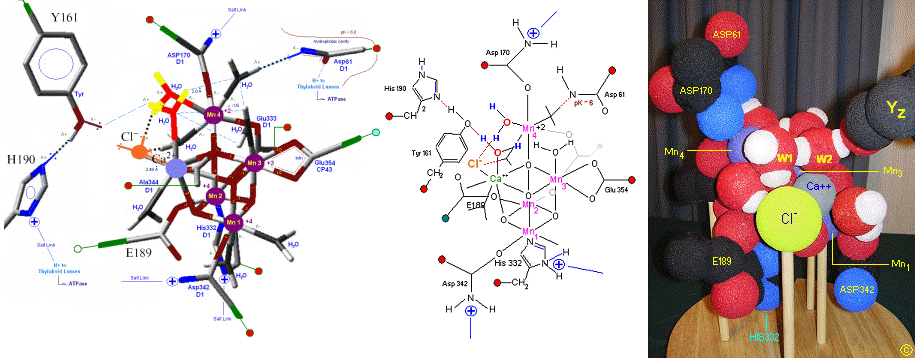
The original X-Ray at a resolution of 3.5 Angstroms, was reported by Ferreira et al in 2004. The image to the left, above, was taken from an article published by James P. McEvoy, Yale University. The image was colored coded and the middle image drawn directly from that. The model on the right was built using this X-Ray information.
The salient features of the complex (not all natural ligands may be in place, and McEvoy added an additional water ligand to complete the complex) include the manganese-calcium oxo bridging of the complex. The manganese atoms are labeled 1, 2, 3 and 4, with manganese number 4 protruding outside the main cubical structure below it, which includes manganese oxo binding, between the Mn 1, Mn 2 and Mn 3. Calcium is placed as one of the cuboidal corners (shown in blue on the left). Chloride is believed to reside on the outside of this cubane complex, and in our model on the right, appears to sit nicely (electrostatically) amongst the water molecules and calcium that surround it.
Yz, the primary electron acceptor of the OEC, sits to the left, approximately 3.4 Angstroms away from the chloride. Note the numerous ligands supporting the complex, which includes several Aspartates (contributed from the nearby D1 protein) as well as a histidine residue (near the bottom), also from D1. The substrate water molecules, labeled W1 and W2 in our model (right) is, at this time, an arbitrary assignment since the exact details of the mechanism is not yet known (we chose these water molecules after the model was built because of their proximity to Mn 4, and to illustrate a potential source of oxygen in our mechanistic scheme below. A proposed water channel may be directed out from aspartate 170 (D1) since it is believed that Tyrosine and histidine (left of the manganese cube) reside in a hydrophobic environment. The argument adapted here, though not experimentally supported at this time, supposes Yz's location adjacent to one of the reactive waters (for proton removal in the scheme below) places it close enough to a water channel for the release of protons during water oxidation. See the reference for Dr. Johannes Messinger below for a review of currently proposed mechanism for water oxidation.
5. A proposed mechanism for the oxidation of water by the OEC of PSII
The following mechanism of water oxidation [outlined below - two alternative paths are given] is derived simply from the physical model above, and a few tips from the literature (see below). The purpose of this section of the MOTM page is to demonstrate to biochemistry students who have had one or two semesters in biochemistry that by using simple Lewis structures, potential mechanistic paths can be discovered through trial and error. While waiting for further research to emerge, a student can familiarize themselves with an enzymatic reaction and get their feet wet simply by sitting down with pen and paper, perhaps a crude model, and make an attempt to deduce a pathway for catalysis. In this way a student may familiarize him or herself with the catalytic problem at hand.
The proposed reaction below is based entirely on the physical model built, along with the X-Ray image above. From the literature the fact that a potential attack on a Mn(V)=O
(manganese oxo) and a proton release pattern of (So![]() S1) 1, (S1
S1) 1, (S1![]() S2)
1, (S2
S2)
1, (S2![]() S3) 0, (S3
S3) 0, (S3![]() S4) 2,
is considered. Aside from this information, the following mechanism was derived simply by applying Lewis dot structures to the catalytic event (reference to the catalase class of
enzymes gives insight into the mechanism of oxo attack by water). Some of the leading mechanisms now proposed may be found in Johannes Messinger's review article, cited below, entitled
Evaluation of different mechanistic proposals for water oxidation in photosynthesis on the basis of Mn4OxCa structures for the catalytic site and spectroscopic data, Phys. Chem. Chem. Phys., 6:4764-4771 (2004)
S4) 2,
is considered. Aside from this information, the following mechanism was derived simply by applying Lewis dot structures to the catalytic event (reference to the catalase class of
enzymes gives insight into the mechanism of oxo attack by water). Some of the leading mechanisms now proposed may be found in Johannes Messinger's review article, cited below, entitled
Evaluation of different mechanistic proposals for water oxidation in photosynthesis on the basis of Mn4OxCa structures for the catalytic site and spectroscopic data, Phys. Chem. Chem. Phys., 6:4764-4771 (2004)
For the purposes of this exercise the following assumptions are made: (1) only 1 Manganese is redox active (Mn number 4 -
most models involve at least two manganese and base their argument on the oxidative potential necessary to carry out the final step of water oxidation, but note too that
the invovlement of Mn 3 or even Mn 1 and Mn 2, does not necessarily require their full oxidation for catalysis);
(2) Tyrosine 161 (Yz) removes protons from the reaction center at every step through the Kok S-state cycle (note that the proton release
pattern, not shown in the Kok diagram above, is believed to be 1 proton from So![]() S1, no protons from
S1
S1, no protons from
S1![]() S2, 1 proton from S2
S2, 1 proton from S2![]() S3,
and 2 protons from S3
S3,
and 2 protons from S3![]() S4; (3) there is an attack on a Mn=0 oxo linkage;
[Note: although not reported, one might envision a Mn triple bond with oxygen - at least one metallo-oxygen evolving scheme uses this] (4) a peroxide
is formed at the S3 state; (5) one substrate water forms a ligand to Ca++ (6) one substrate water is
forms a ligand to Mn 4 (5 and 6 based simply at looking at the physical model. Chloride is assumed to play a co-factor supporting role.
S4; (3) there is an attack on a Mn=0 oxo linkage;
[Note: although not reported, one might envision a Mn triple bond with oxygen - at least one metallo-oxygen evolving scheme uses this] (4) a peroxide
is formed at the S3 state; (5) one substrate water forms a ligand to Ca++ (6) one substrate water is
forms a ligand to Mn 4 (5 and 6 based simply at looking at the physical model. Chloride is assumed to play a co-factor supporting role.
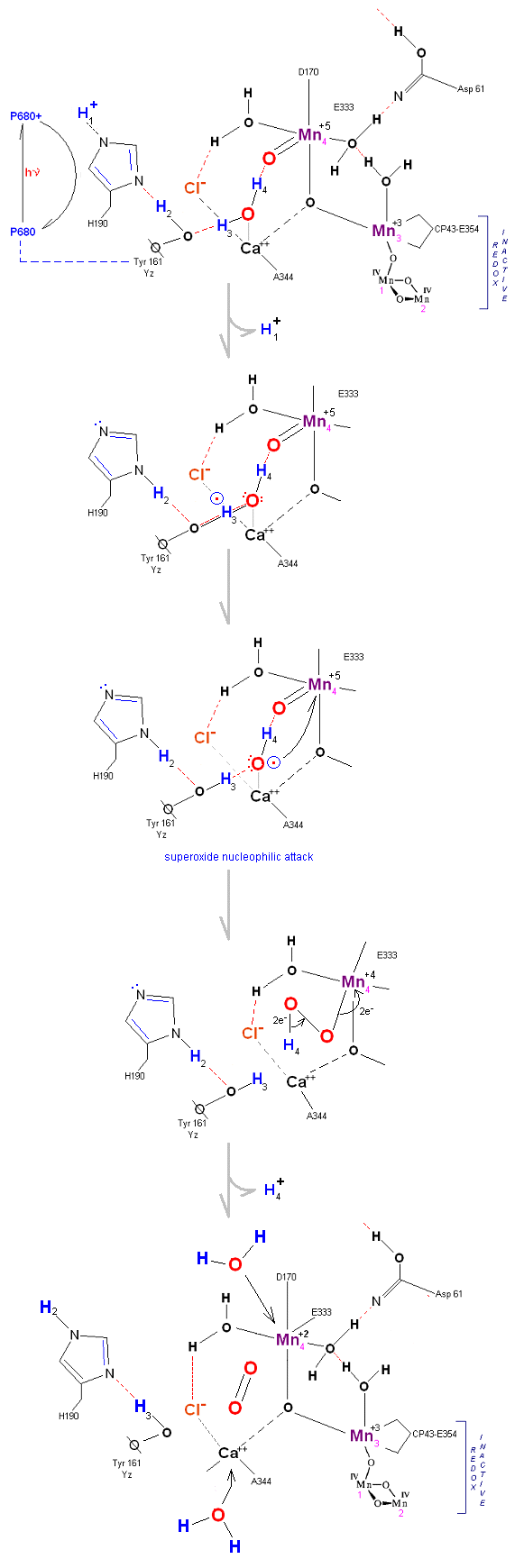
Recent X-Ray data suggests that protons are removed (via a water channel) on the Asp 61 side of the OEC (the argument being that Yz (Y161) is in a hydrophobic pocket and removed from what appears to be a channel to the lumen on the Asp 61 side of the complex). Since the mechanism proposed below requires Yz to extract protons with incoming photons, the protons are modeled here as being removed on the Yz side of the OEC complex (its just a model!).
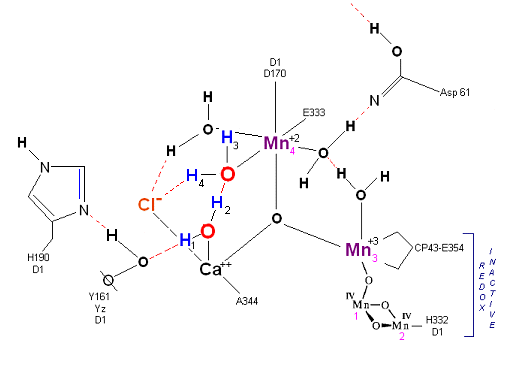
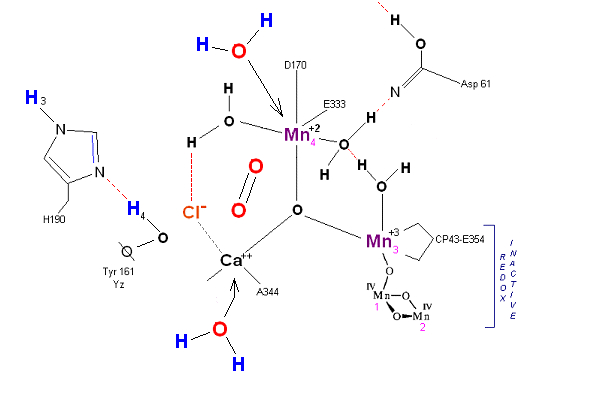
State So: Resting state. The two substrate water are colored. Mn 4 begins in a +2 state. Mn 3, Mn 2 and Mn 1 are in a +3, +4, +4 state (and remain that way being redox inactive for this scheme). Only Mn 1 is redox active. Protons are numbered 1-4. Note that the two substrate water molecules were chosen simply based on their suggestive positions from the physical model above. No argument is made here as to D1 ligand changes (for example, Mn in its lower oxidation state may prefer a hexagonal arrangement and may move to pentagonal coordination as its oxidation step increases but this is not considered here). Photon arrives at P680).
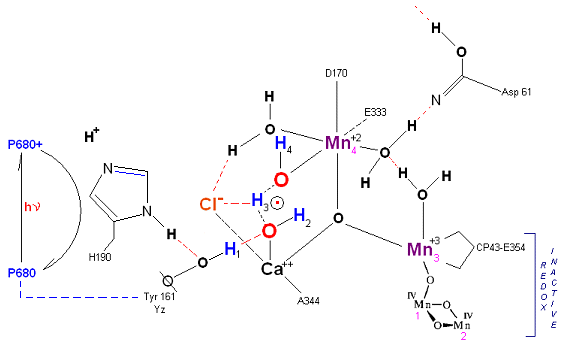
State So': First photon arrives. P680+ reduced by Yz. Initial + charged carried by hydrogen bridge between Mn 2 and Ca (note that in the physical model this transitory bridge would be linear). Cl- helps to stabilize the positive charge. Notice that in a one proton extraction scenario such as this Histidine 190 may spin 180 degrees before resting in the S1 state (alternatively a simple 2e- resonance action could result in relaxation to S1).
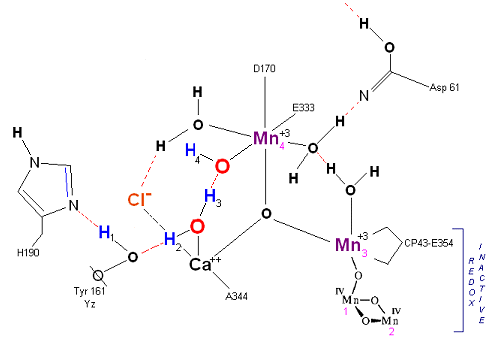
State S1: S1 resting state. H3 transfers from substrate H2O #2 to H2O #1. Redox reactive Mn 4 is now in the Mn(III) oxidation state. Next photon arrives at P680.
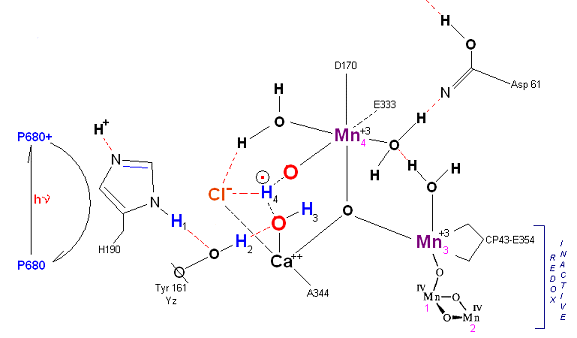
State S1': P680+ is reduced by Yz. Hydrogen bridge forms as in So![]() S1, charged stabilized by Cl-.
S1, charged stabilized by Cl-.
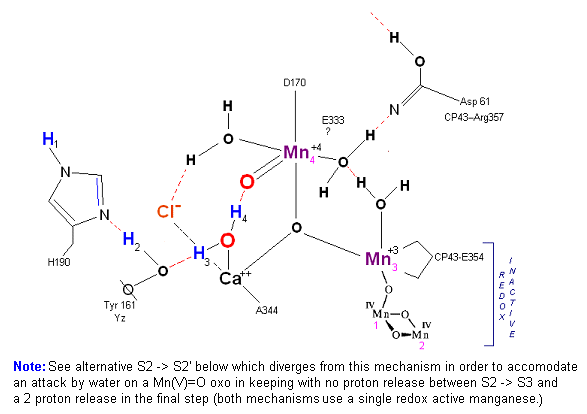
State S2: S2 resting state. Mn 4 is now an oxo-manganese (carbonyl) as Mn(IV), with a double bond to the original substrate H2O #2. Ligands around Mn 4 may shift here. Cl- still serving in a stabilizing fashion. Note that the Tyrosine-Histidine system is moving protons out one at a time. Next photon arrives at P680.
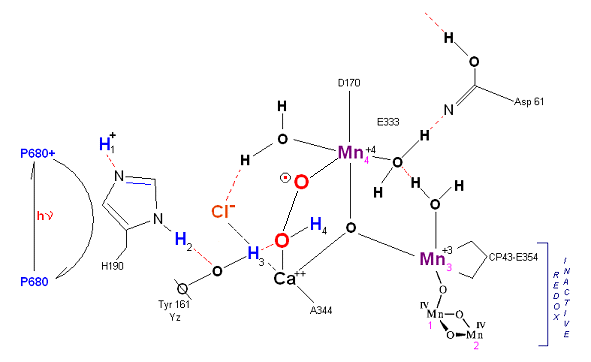
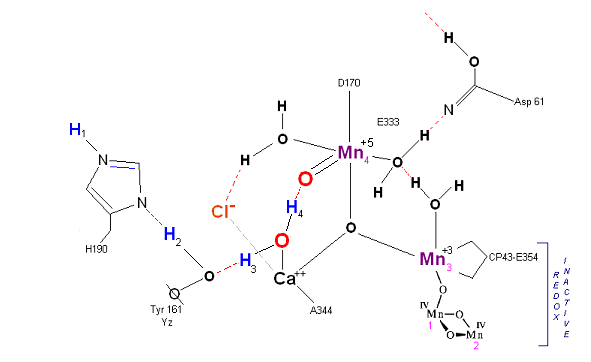
State S2': P680+ is reduced. This time the intermediate is a peroxo-radical. The move to the resting S3 state involves the reduction of Mn 4 from Mn(IV) to Mn(III) It is possible that H4 may bridge the two oxygens to stabilize the radical. [Note: See alternative mechanism below in which a Mn(V)=O is created which is attacked by water in the S3 ![]() S4 transition (this accommodates the proton release pattern of 1,0,1,2 as referenced in the literature)].
S4 transition (this accommodates the proton release pattern of 1,0,1,2 as referenced in the literature)].
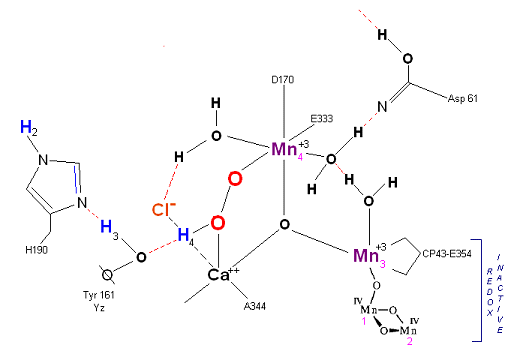
State S3: S3 resting state is a stable peroxide. Mn 4 is now back to a Mn(III) oxidation state. Only one of the original Hydrogens remained bound. Next photon arrives at P680.
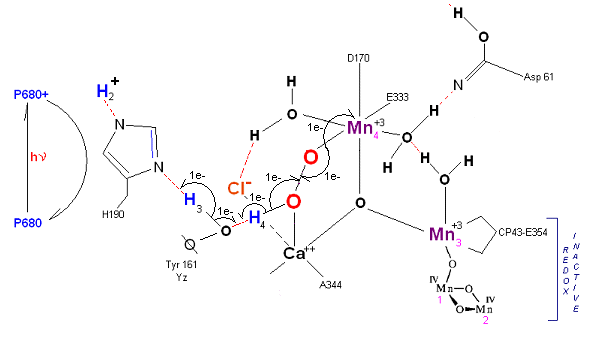
State S3' ![]() S4: Finally in a series of 1 electron transfers, following the reduction of P680, Mn(III) returns to
its ground state Mn(II), oxygen is formed, and the system prepares itself to be reset to the initial State So.
S4: Finally in a series of 1 electron transfers, following the reduction of P680, Mn(III) returns to
its ground state Mn(II), oxygen is formed, and the system prepares itself to be reset to the initial State So.
State S4 ![]() S4':
S4':
Incoming water molecules resets the OEC to State So.
Alternative route (water attack on Mn(V)=O releasing 2H+ from S3 ![]() S4). In this scenario S2' releases an electron form Mn 4 but no protons, the resultant S3 state from S2 is shown below.
S4). In this scenario S2' releases an electron form Mn 4 but no protons, the resultant S3 state from S2 is shown below.
State S2 ![]() S3:
S3:
In this alternative mechanism only an electron is removed in the S2 ![]() S3 transition accommodating the argument that no proton is released as well as the
final step involving a direct attack by water on a Mn(V)=O complex (this mechanism is found in several oxygen evolving enzymatic scenarios).
S3 transition accommodating the argument that no proton is released as well as the
final step involving a direct attack by water on a Mn(V)=O complex (this mechanism is found in several oxygen evolving enzymatic scenarios).
References
1.Lowe, D. R., Early Life on Earth, Nobel Symposium No. 84, ed., Bengtson, S., Columbia Univ. Press, New York, 24-35 (1994)
2. Paul Preuss, Spinach, Or The Search For The Secret Of Life, Science Beat, July 2004.
3. McEvoy, James P., Gascon, Jose A. et al.,
Computational Structural Model of the Oxygen Evolving Complex in Photosystem II: Complete Ligation by Protein, Water and Chloride, Photosynthesis:
Fundamental Aspects of Global Perspectives, A. van der Est and D. Bruce, Eds., 278-80 (2005)
4. Michael Russell, First
Life, The American Scientist 94:1,32 Jan-Feb (2006)
5. Todar, Kenneth, Major Groups of Prokaryotes, University of Wisconsin-Madison, Department of Bacteriology (2004)
6. Schwartz, Edward and Friedrich, Barbel, The H2-Metabolizing Prokaryotes [References available]
7. Earth's Early Years: Differentiation, Water and Early Atmosphere, University of Michigan
8. Fegley, Calculations favor reducing atmosphere for early Earth, Washington University, St. Louis (2005)
9. Rasmussen, Birger, Filamentous microfossils in a 3,245-million-year-old volcanogenic massive sulphide deposit. Nature 405 676-679 (June 2000), Dept. of Geology and Geophysics, University of
Western Australia, Nedlands, Western Australia 6907, Australia
10. Woese, C.R. Towards a natural system of organisms: Proposal for the domains Archaea, Bacteria, and Eucarya. Proc. Natl. Acad. Sci. 87:4576-4579 (1990).
11. Russell, M. J., Daniel, R. M., Hall, A. J., Sherringham, J. A., J. Mol. Evol., 39:231-43 (1994)
12. Anbar, A. D., Holland, H. D. Geochim. Cosmochim. Acta, 56:2595-2603 (1992)
13. Dawson, Scott, Creatures
from the Black Lagoon: Lessons in the Diversity and Evolution of Eukaryotes, Berkeley University, 1-18
14. Brandes, Jay and Hazen, Bob, Key chemical in life creation - ammonia - created at Hydrothermal Vents. Carnegie Institution's Geophysical Laboratory (1998)
15. Blank, Biologist's Find Alters The bacteria Family Tree, Science Daily, Department of Earth & Planetary Sciences at Washington University in St. Louis
16. Konhauser, Kurt ). et al., Could bacteria have formed the Precambrian banded iron formations?, Geology 30:12, 1079-82 (2002), School of Earth Sciences, University of Leeds
17. Univeristy of Michigan, Earth's Early Years: Water and Early Atmosphere
18. Levy, Dawn, Rocks tell tale of warm early atmosphere, Stanford Report, June 2004.
19. Sankaran, A. V., The Controversy over early-Archaean microfossils, Current Science, 83:15-17 (2002).
Photosystem II - General references
1. Barber, James, Towards a full understanding of water splitting in photosynthesis, International Journal of Photoenergy6:43-51 (2004)
2. Ferreira, Kristina, Iverson, Tina, Maghlaoui, Karim, Barber, James,
Architecture of the Photosynthetic Oxygen-Evolving Center, Science 303:1831-1837 (2004)
3. Govindjee and Krogmann, David,
Discoveries in oxygenic photosynthesis (1727-2003): a perspective, Photosynthesis Research 80:15-57 (2004)
4. Govindjee and Orr, Larry, Photosynthesis on the Web, Departments of Biochemistry and Plant Biology and Center
of Biophysics & Computational Biology, University of Illinois, Urbana, IL and Center for the Study of Early Events in Photosynthesis,
Arizona State University respectively (2005)
5. Govindjee, J. T. Beatty, H. Gest, and J. F. Allen, eds., Advances in Photosynthesis and Respiration Vol. 20: Discoveries in Photosynthesis,,
Springer, Dordrecht (2005). pp. 119-129
6. Govindjee, J. T. Beatty, and H. Gest eds., special issue: Celebrating the Millennium—Historical Highlights of Photosynthesis Research, Part 2, Photosynthesis Research 76:93-103
7. Crofts, Anthony, Structure and Function in Photosystem II, Crofts Laboratory, University of Illinois
8. Haumann, Micahel, and Junge, Wolfgang,
Photosynthetic Water Oxidation: A Simplex-Scheme of its Partial Reactions, Biochem. Biophys. Acta 1411:1-6 (1999)
9. Hillier, Warwick and Wydrzynski, Tom, The Affinities for the Two Substrate Water Binding Sites in the O2 Evolving
Complex of Photosystem II Vary Independently during S-State Turnover, Biochemistry 200, 39:4399-4405 (2000)
10. J.P. and Shock, E.L., Energetics of overall metabolic reactions in
thermophilic and hyperthermophilic Archaea and bacteria. FEMS Microbiology Reviews 25:175-243 (2001)
11. Kok, B., B. Forbush, and M. McGloin. 1970. Cooperation of charges in photosynthetic
oxygen evolution. I. A linear four step mechanism. Photochem. Photobiol. 11:467–475.
12. McEvoy, James P., Gascon, Jose A. et al.,
Computational Structural Model of the Oxygen Evolving Complex in Photosystem II: Complete Ligation by Protein, Water and Chloride, Photosynthesis:
Fundamental Aspects ot Global Perspectives, A. van der Est and D. Bruce, Eds., 278-80 (2005)
13. Messinger, Johannes, Evaluation of different mechanistic proposals for water oxidation in photosynthesis on the basis of Mn4OxCa structures for the catalytic site and
spectroscopic data, Phys. Chem. 6:4764-4771 (2004)
14. Zouni, Athina, Horst-Tobias Witt, Kern, Jan et al. Crystal structure of Photosystem II from Synechococcus elongatus at 3.8 Angstrom resolution, Nature 409:739-743 (2001)
15. Homann, P. H., Hydrogen metabolism of green algae. Discovery and early research - a tribute to Hans Gaffron and his coworkers. Invited contribution. (2003)
16. ASU Center for the Study of Early Events in Photosynthesis,
Photosynthesis Center, AZ State Univ.
17. Des Marais, David J., Evolution: When Did Photosynthesis Emerge on Earth?, Science, NASA Ames Research Center, Moffett Field, CA
18. Rutherford and Boussac, Biochemistry: Water Photolysis in Biology, 1782-1784 (2004)
19. Cook, Steve, Steve's Place - Photosynthesis (and other biochem subjects) - an academic site for students.
20. Kondratieva, Elena N., Pfennig, Norbert, Truper, Hans G., the Phototrophic Prokaryotes, from The Prokaryotes, an Evolving Electronic Resource (1991-2006).
Have a comment?
We are creating a "Teaching with Molecular Models Weblog" (www link depository)
The Weblog is under construction and should be ready by this fall (2006). Drop by!
![]()