
Low Level Ozone:
Introduction
Ozone is present in the lower atmosphere at ground level although it is a toxic gas it is considered to be safe in quantities not exceeding 30ppb (parts per billion). A number of human activities are responsible for increasing the level of ozone concentration in the air to a level where it can be dangerous. Low level ozone pollution is often not given much consideration next to the depletion of the ozone layer. However, it can damage health of humans, animals, trees and plants. In high quantities it also contributes to acid rain and the green house effect, as well as being partly responsible for photo chemical smog.
Photochemical Smog
Ozone is a by product of fossil fuel combustion. When pollutants such as hydrocarbons and nitrogen oxides react with sunlight, to produce ozone following a series of free radical reactions. Photochemical smog is a complex pollutant generated when hydrocarbons and oxides of nitrogen react in strong sunlight, forming a series of secondary pollutants.
The major components of photochemical smog are:
- Ozone
- Peroxyacyl nitrates
- Aldehydes
- Alkyl Nitrates
- Air borne particles
The formation of a photochemical smog is typically initiated by photolysis of nitrogen dioxide, which leads to the production of ozone and in turn the reformation of NO2. This is one of the most important photochemical reactions in the lower atmosphere: Return to Top
Low Level Ozone Synthesis:
NO2 + hv  NO + O (1)
NO + O (1)
When nitrogen dioxide is excited by light nitric oxide and atomic oxygen are formed. The rate of this equation is k1[NO2]. k1 is the rate coefficient for this reaction and is first order. The rate of the reaction is not only proportional to the concentration of nitrogen dioxide. It also depends on the intensity of light. At night k1 approaches 0 and reaches its maximum during hours of high sunlight intensity.
The first reaction of the atomic oxygen tends to be with the more abundant diatomic oxygen (O2)
O2 + O + M O3 + M (2)
O3 + M (2)
M is an unreactive 3rd molecule which is needed to absorb excess energy from the reaction. The rate coefficient of this reaction is k2. There is a third and final reaction which completes this this reaction:
O3 + NO  NO2 + O2 (3)
NO2 + O2 (3)
This reaction has the rate coefficient k3.
Each of the 3 reactions is very fast, and a photostationary state of equilibrium is established, this governs the ratio of NO2/NO in air, which in turn can be shown to be proportional to the concentration of ozone.
[NO2]/[NO] = k3[O3]/k1 (4)
So as k1approaches 0 (night time conditions. The presence of excess ozone increases and the lowest [NO2]/[NO] ratios are during hours of extreme day light.
The reactions (1) and (2) produce ozone and mono-atomic oxygen, which go on to react with
hydrocarbons released from fossil fuels. Alkenes are particularly susceptible to attack from these
oxygen allotropes. A typical reaction is shown below:
CH3CH=CHCH3+O3
 CH3CHO2+CH3CHO(5)
CH3CHO2+CH3CHO(5)
The reaction chain is then propagated:
C2H5+O2
 C2H5O2(6)
C2H5O2(6)C2H5O2+NO
 C2H5O+NO2(7)
C2H5O+NO2(7)C2H5O+O2
 CH3CHO+HO2(8)
CH3CHO+HO2(8)HO2+NO
 NO2CHO2+OH(9)
NO2CHO2+OH(9)
Reactions (7) and (9)convert NO to NO2 without the use of ozone, keeping NO concentrations reasonably low. Reaction 3 is relatively unimportant meaning that high levels of ozone are able to build up. Photochemical smog's are a particular problem in built up cities where there are high numbers of vehicles and industries relying on combustion of fossil fuels. Eventually winds disperse the smog.
A profile of the concentrations of the pollutants in photochemical smog, and how they vary throughout a day are shown below, note the relative amounts at different levels of sunlight:
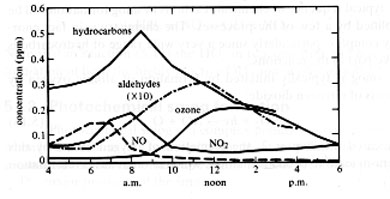
Acid Rain
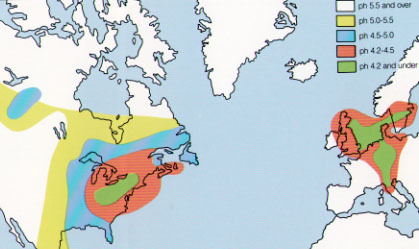
Acid rain is a very well publicised environmental problem, but it is a less known fact that low level ozone plays a role in pollution of this kind. Ozone aid the oxidation of NO2 to nitric acid, and convert HSO3- (from SO2) to HSO4-. In solution in rain water these acids can cause acid rain with pH4 or lower. The resulting acid water vapour can be carried in the winds and fall causing damage to trees, buildings and water life. Western Europe has witnessed particularly large damage and death due to acid rain. The map below demonstrates this clearly:
Health of Organisms
Low level ozone also presents a problem to health. It is a toxic gas on inhalation and some countries governments have issued warnings. The world health organisation recommends a maximum hourly dose of 80ppb. It is not recommended to participate in strenuous exercise and athletic competitions have been cancelled in the past. Ozone is also produced by some electrical devices, photocopier's distinct smell is down to ozone.
Some symptoms are shown in the table below:
| Ozone Levels Hourly ppb | Symptoms |
| 50 | Headaches |
| 150 | Eye Irritation |
| 270 | Coughs |
| 290 | Chest Discomfort |
Ground level ozone also affects plants. Most plants exchange gases with the surrounding air through stomata. These holes also let in ozone and affect cells within the plant. Mitochondria and chloroplasts are affected, preventing the plant to photosynthesise and respire effectively. The plant leaves develop spots, go brown, then the plant dies. The tobacco plant Nicitiana is particularly susceptible to ozone and has been used to monitor ozone levels and patterns nation-wide.
Return to Top
| THE CHEMICAL OZONE | HIGH LEVEL OZONE |
| INFO. ON ME | LINKS |