
OZONE CHEMISTRY:
This site aims to provide information on the chemistry of ozone and provide some useful links to other pages.
Ozone is an atmospheric compound, found both at ground level and in the stratosphere. It also is
responsible for various chemical reactions. Ozone is crucial for life as we know it, preventing
harmful radiation from reaching the earth. But, it also has a not so nice side. Low level ozone is
partly responsible for photochemical smog's in our cities and also presents a health hazard. This
site will give you the low down on low and high level ozone, as well as having a look at the ozone
hole and the chemistry behind this unique compound.

Ozone Factfile
- Standard State: gas
- Boling temperature: -111.9 °C
- Melting temperature: -195.5 °C
- Specific Gravity: 2.144
- Formula: O3
- Colour: Pale Blue
- Occurs: naturally in troposphere
- Formed: From nitrogen oxides and organic oxides emitted as a product of combustion engines or from passing an electric current through air.
The name ozone comes from the Greek ozein, for "to smell". It is an allotropic form of oxygen having three atoms in each molecule, formula O3.
It is, in standard state, a pale blue, highly poisonous gas with a strong odour. Liquid ozone is a deep blue and strongly magnetic.
Ozone is formed when an electric spark is passed through oxygen, and causes a detectable odour near electrical machinery. The commercial method of preparation consists of passing cold, dry oxygen through a silent electrical discharge.
Ozone is much more active chemically than ordinary oxygen and is a better oxidising agent. It is used in purifying water, sterilising air, and bleaching certain foods.
Ozone formed in the atmosphere is from nitrogen oxides and organic gases emitted by automobiles and industrial sources, however, is a health hazard, and it may cause serious crop damage in some areas.
The structure of ozone has 3 oxygen atoms, but steric hindrance prevents it from forming a triangular structure, with each O atom forming the expected 2 bonds. Instead each Oxygen forms only 1 bond, with the remaining negative charge being spread throughout the molecule.

Ozone Reactions
Ozone reactions mostly take place in the atmosphere, as the unstable molecule reacts in sunlight. These are described in Low Level Ozone and High Level Ozone. There are a group of compounds called Ozonides, which are formed during the reaction of ozone with alkali metal hydroxides, formally containing the O3- ion. An ozonide is also the unstable compound formed by the addition of ozone to the C=C bond in alkenes. Ozone is also a very strong oxidising agent. This is shown below:
2Fe2++2H++ O3  2Fe3++H2O+O2
2Fe3++H2O+O2
Ozonolysis is a reaction of alkenes with ozone. Ozone can be produced in the lab using a machine called an ozonator. This ozone is passed through a solution of the alkene, first producing an ozonide. This ozonide is subsequently reduced, giving the result of cleavage around the double bond, with an oxygen attached to each carbon from the double bond. The reaction is shown below:
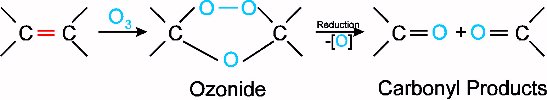
It becomes apparent that as well as being important in environmental chemistry, ozone also plays a role in organic chemistry.

High Level Ozone:
Introduction
The majority of ozone in the earth's atmosphere is found in the stratosphere(15-50km above the earth's surface) This high level ozone plays a crucial role, protecting animals and plants from the suns harmful ultra violet rays, and stabilising the earth's climate. This ozone layer is incredibly unstable, since it is constantly being formed and broken down through interactions with UV radiation. But, as it is so reactive the ozone layer can easily be broken down by pollutant gases rising from the earth. CFC's and nitrous oxide are thought to be particularly responsible for the breakdown of the ozone layer. Since the hole in the ozone layer has developed cases of skin cancer, particularly have grown.
The Role of the Ozone Layer
The primary role of the ozone layer is to prevent the sun's harmful ultra violet rays from reaching life on earth. This UV radiation is of a suitable energy to damage living cells, causing sunburn which can lead to skin cancer. The areas of the planet with thinner ozone coverage have much higher incidences of skin cancer. This is particularly true around the equator. It is thought that a reduction of 1% of the ozone in the stratosphere could lead to:
- An increase in skin cancers in animals and humans
- A suppression of the human immune systems
- Some inhibition to plant life, and an increased susceptibility to pests
- Reduction in growth of phytoplankton, endangering the food chain
- A decrease in aquatic lifeforms
Ozone absorbs UV radiation in its formation and breakdown: O2absorbs UV light to form 2 oxygen radicals:
O2 + hv  O + O (1)
O + O (1)
O+O2 O3 (2)
O3 (2)
O+O O2 (3)
O2 (3)
O+O3  2O2 (4)
2O2 (4)
O3 + hv  O2 + O (5)
O2 + O (5)
Ozone in the stratosphere also plays a role in absorbing some of the infra-red radiation which travels from the earth into space, keeping the earth warm. In this role it is partly responsible for global warming. The primary culprits for this, however, are the pollutant gases from human activities which seem to exaggerate the effect, making the earth too warm.

The Ozone Hole:
In the early 1980's scientists who had been studying the atmosphere above the Antarctic realised that ozone was disappearing from the area every spring. Data showed that the total amount of ozone in the atmosphere declined by 2% between 1978 and 1985. This may not seem much, but ozone is a trace element in the stratosphere maintaining a delicate balance. There are only a few parts of ozone per billion.
Why were ozone levels decreasing? The answer came when Chlorofluorocarbons, were found in the stratosphere. These chemicals had originally been developed because they were so stable. They were used as refrigerants in air conditioners and fridges, Blowing agents in expanded plastics(e.g. polystyrene), Aerosol Propellants, and Cleaning Solventsto dissolve grease, particularly in dry cleaning. The stability of these gases mean that they can exist in the atmosphere for as long as 75 years. During this time they can be carried by winds into the stratosphere. It is here where they breakdown, due to the sun's high energy radiation, into hydrogen fluoride and chlorine. It is the chlorine and the fluorine which are responsible for the destruction of the ozone layer. Nitrogen oxides are also thought to be partly responsible for the decay of the ozone layer, but they can also rebuild it as shown by the equations in Low Level Ozone.
The Breakdown of Ozone:
Here chlorine will be used as the example: When CFC's are destroyed they form Cl radicals. These chlorine radicals react with ozone:
Cl+O3 O2+ClO (6)
O2+ClO (6)
ClO+O Cl+O2 (7)
Cl+O2 (7)
O+O2 O3 (2)
O3 (2)
The hole in the ozone layer is particularly bad over Antarctica, although most of the CFC's are released in the Northern Hemisphere. This is because winds blow the CFC's to the poles. Below is a graph of the October levels of ozone above the Antarctic between 1980 and 1991:
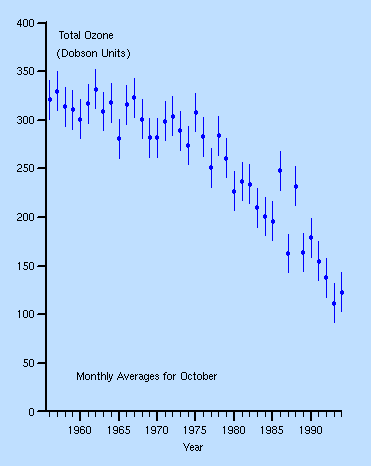
The reduction over time is clear. The pictures corresponding to the graph are below. They show how the ozone hole has grown over the past years. It is thought to continue growing until about 20 years after the complete phasing out of CFC's.
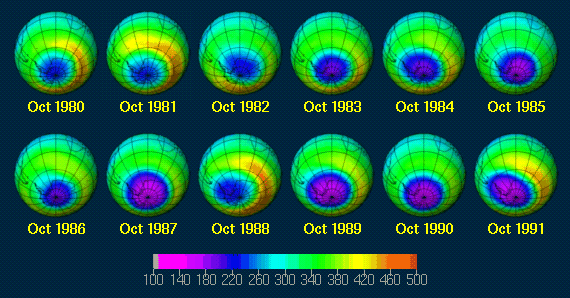
After much research by scientists all over the world governments decided that something had to be done to stop the ozone depletion of the ozone layer. In 1987 24 countries signed a treaty aiming to save the ozone layer. This Montreal Protocol called for a reduction in the production of ozone depleting substances, aiming to reduce CFC's by 50% by the year 2000. Since then alternatives have been found to fulfil the roles of CFC's and the amounts being released to the atmosphere have dramatically reduced. However the ozone hole is still a problem needing to be carefully monitored.

Low Level Ozone:
Introduction
Ozone is present in the lower atmosphere at ground level although it is a toxic gas it is considered to be safe in quantities not exceeding 30ppb (parts per billion). A number of human activities are responsible for increasing the level of ozone concentration in the air to a level where it can be dangerous. Low level ozone pollution is often not given much consideration next to the depletion of the ozone layer. However, it candamage health of humans, animals, trees and plants. In high quantities it also contributes to acid rain and the green house effect, as well as being partly responsible for photo chemical smog.
Photochemical Smog
Ozone is a by product of fossil fuel combustion. When pollutants such as hydrocarbons and nitrogen oxides react with sunlight, to produce ozone following a series of free radical reactions. Photochemical smog is a complex pollutant generated when hydrocarbons and oxides of nitrogen react in strong sunlight, forming a series of secondary pollutants.
The major components of photochemical smog are:
- Ozone
- Peroxyacyl nitrates
- Aldehydes
- Alkyl Nitrates
- Air borne particles
The formation of a photochemical smog is typically initiated by photolysis of nitrogen dioxide, which leads to the production of ozone and in turn the reformation of NO2. This is one of the most important photochemical reactions in the lower atmosphere:
Low Level Ozone Synthesis:
NO2 + hv  NO + O (1)
NO + O (1)
When nitrogen dioxide is excited by light nitric oxide and atomic oxygen are formed. The rate of this equation is k1[NO2]. k1 is the rate coefficient for this reaction and is first order. The rate of the reaction is not only proportional to the concentration of nitrogen dioxide. It also depends on the intensity of light. At night k1 approaches 0 and reaches its maximum during hours of high sunlight intensity.
The first reaction of the atomic oxygen tends to be with the more abundant diatomic oxygen (O2)
O2 + O + M O3 + M (2)
O3 + M (2)
M is an unreactive 3rd molecule which is needed to absorb excess energy from the reaction. The rate coefficient of this reaction is k2. There is a third and final reaction which completes thisthis reaction:
O3 + NO  NO2 + O2 (3)
NO2 + O2 (3)
This reaction has the rate coefficient k3.
Each of the 3 reactions is very fast, and a photostationary state of equilibrium is established, this governs the ratio of NO2/NO in air, which in turn can be shown to be proportional to the concentration of ozone.
[NO2]/[NO] = k3[O3]/k1 (4)
So as k1approaches 0 (night time conditions. The presence of excess ozone increases and the lowest [NO2]/[NO] ratios are during hours of extreme day light.
The reactions (1) and (2) produce ozone and mono-atomic oxygen, which go on to react with hydrocarbons released from fossil fuels. Alkenes are particularly susceptible to attack from these oxygen allotropes. A typical reaction is shown below:
CH3CH=CHCH3+O3
 CH3CHO2+CH3CHO(5)
CH3CHO2+CH3CHO(5)
The reaction chain is then propagated:
C2H5+O2
 C2H5O2(6)
C2H5O2(6)C2H5O2+NO
 C2H5O+NO2(7)
C2H5O+NO2(7)C2H5O+O2
 CH3CHO+HO2(8)
CH3CHO+HO2(8)HO2+NO
 NO2CHO2+OH(9)
NO2CHO2+OH(9)
Reactions (7) and (9)convert NO to NO2 without the use of ozone, keeping NO concentrations reasonably low. Reaction 3 is relatively unimportant meaning that high levels of ozone are able to build up. Photochemical smog's are a particular problem in built up cities where there are high numbers of vehicles and industries relying on combustion of fossil fuels. Eventually winds disperse the smog.
A profile of the concentrations of the pollutants in photochemical smog, and how they vary throughout a day are shown below, note the relative amounts at different levels of sunlight:
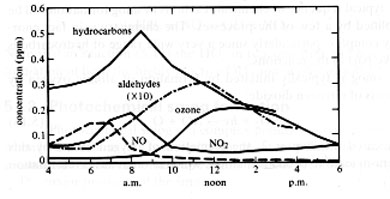
Acid Rain
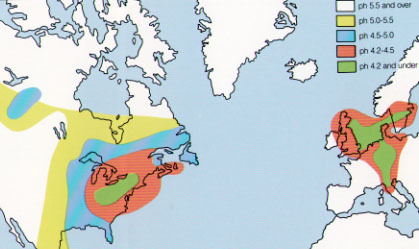
Acid rain is a very well publicised environmental problem, but it is a less known fact that low level ozone plays a role in pollution of this kind. Ozone aid the oxidation of NO2 to nitric acid, and convert HSO3- (from SO2) to HSO4-. In solution in rain water these acids can cause acid rain with pH4 or lower. The resulting acid water vapour can be carried in the winds and fall causing damage to trees, buildings and water life. Western Europe has witnessed particularly large damage and death due to acid rain. The map below demonstrates this clearly:
Health of Organisms
Low level ozone also presents a problem to health. It is a toxic gas on inhalation and some countries governments have issued warnings. The world health organisation recommends a maximum hourly dose of 80ppb. It is not recommended to participate in strenuous exercise and athletic competitions have been cancelled in the past. Ozone is also produced by some electrical devices, photocopier's distinct smell is down to ozone.
Some symptoms are shown in the table below:
| Ozone Levels Hourly ppb | Symptoms |
| 50 | Headaches |
| 150 | Eye Irritation |
| 270 | Coughs |
| 290 | Chest Discomfort |
Ground level ozone also affects plants. Most plants exchange gases with the surrounding air through stomata. These holes also let in ozone and affect cells within the plant. Mitochondria and chloroplasts are affected, preventing the plant to photosynthesise and respire effectively. The plant leaves develop spots, go brown, then the plant dies. The tobacco plant Nicitiana is particularly susceptible to ozone and has been used to monitor ozone levels and patterns nation-wide.


Links:
These links are to various pages on the subject matter of ozone. They contain a wide range of subject matter and links. Most of the information is relavent to the information carried within this document.
- University of Bristol Chemistry Department.
- Pages on ozone, from the US environmental agency
- Ozone multimedia tour, from Cambridge University
- The Environmental effects of ozone depletion
- Ozone data, British Antarctic Survey
- The Ozone; its past, present and future
- Panic Ozone, parallel to Alice through the Looking Glass
- Ozone, chemical of the week
- KNMI Ozone home page
- Common Questions on Ozone
- Atmospheric Chemistry data and resources, from the Goddard space flight center
- Oh-Oh Ozone, Nancy Lockhoff, Austin Clean Air Force
- Atmospheric Chemistry, the ozone layer links
- Basic Chemistry of Ozone depletion
- UK environment agency, low level ozone
- Low Level Ozone Factfile

Eloise Stattersfield:
I am a first year Chemistry student at the University of Bristol, following the year in industry MSc programme. I have written this page as part of my computer method's course work. To E-mail me (es5145@bris.ac.uk)click here:

This site was last updated 31st August 2001
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5271673]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5271673]