![]()
PHENYLETHYLAMINE (PEA)
and TYRAMINE
(the amine responsible for 'the cheese effect')
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month March 2012
Also available: JSMol version.
![]()
|
PHENYLETHYLAMINE (PEA)and TYRAMINE(the amine responsible for 'the cheese effect')
Simon Cotton
Molecule of the Month March 2012
|
 |
No good at all.
Because the -NH2 group isn't bonded directly to the benzene ring (as it is in phenylamine), the diazonium ion formed from phenylethylamine is not stabilised by interaction with the aromatic π-system. This makes it as unstable as any alkyldiazonium ion.
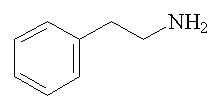
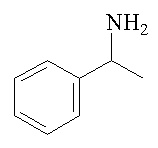
Strictly, it should be called 2-phenylethylamine, or β-phenylethylamine, to distinguish it from its isomer, 1-phenylethylamine, or α-phenylethylamine; this has a chiral carbon atom and thus exists as optical isomers, unlike 2-phenylethylamine.
 |
 |
2-phenylethylamine |
1-phenylethylamine |
2-phenylethylamine (PEA) is a trace amine, found in very small amounts in the human body. It is the parent of the amphetamines, such as methamphetamine; the name "amphetamine" is a contraction of Alpha-Methyl-PHenylEThylAMINE.
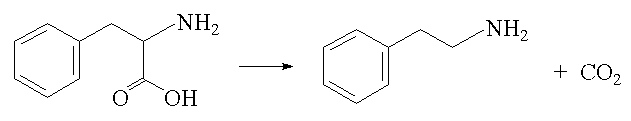
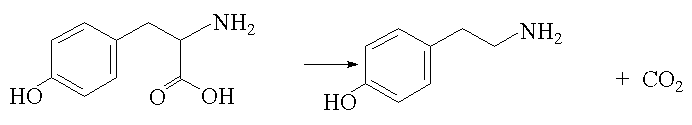
They are also sometimes referred to as microamines, formed in the body by enzymatic decarboxylation of aminoacids, thus phenylethylamine is formed from phenylalanine:
Other trace amines include tyramine, tryptamine, phenethylamine, and octopamine. They are found around the nervous system with concentrations in the range 2-15 nanomolar. In the brain, they are associated with the dopamine, norepinephrine, and serotonin neurotransmitter systems and appear to play a role in the brain's circuits. A new class of G protein-coupled receptor has been discovered, known as trace amine-associated receptors (TAARs), selectively activated by trace amines.
 A study of phenylethylamine levels in the brain of a rat found that administering phenylalanine increased the brain levels of β-phenylethylamine, and that inhibition of monoamine oxidase caused a 40-fold increase in brain β-phenylethylamine. The monoamine oxidase B (MAO-B) enzyme in the body selectively metabolises phenylethylamine to phenylacetic acid, and it has been reported that the concentration of phenylacetic acid is significantly reduced in the urine, plasma and cerebrospinal fluid of depressed patients. Brain concentrations of 2-phenylethylamine are significantly increased by monoamine oxidase inhibitor antidepressants; urinary concentrations of phenylacetic acid are increased following exercise, suggesting that phenylethylamine may be involved in the "runner's high", the state of euphoria associated with a level of physical exercise.
A study of phenylethylamine levels in the brain of a rat found that administering phenylalanine increased the brain levels of β-phenylethylamine, and that inhibition of monoamine oxidase caused a 40-fold increase in brain β-phenylethylamine. The monoamine oxidase B (MAO-B) enzyme in the body selectively metabolises phenylethylamine to phenylacetic acid, and it has been reported that the concentration of phenylacetic acid is significantly reduced in the urine, plasma and cerebrospinal fluid of depressed patients. Brain concentrations of 2-phenylethylamine are significantly increased by monoamine oxidase inhibitor antidepressants; urinary concentrations of phenylacetic acid are increased following exercise, suggesting that phenylethylamine may be involved in the "runner's high", the state of euphoria associated with a level of physical exercise.
When PEA was administered to depressed patients (together with selegiline, a MAO-B inhibitor added to prevent destruction of PEA), the depression was relieved in 60% of cases, leading to the suggestion that a deficit of PEA may be a cause of depression.
 Is it true that chocolate is full of PEA?
Is it true that chocolate is full of PEA?Several studies have shown that PEA is present in cocoa beans and chocolate. Its level increases during fermentation of cocoa and in roasting cocoa beans, with a concentration in the mg per kg range. This led to a widely-touted suggestion that people could increase their brain levels of PEA by eating chocolate, and that this could be linked with falling in love (even nowadays, especially around February 14th). This may have done wonders for chocolate sales, but the rapid metabolism of phenylethylamine by MAO-B will stop this PEA from reaching the brain.
 Does PEA affect other animals?
Does PEA affect other animals?It's recently been discovered that carnivorous animals such as lions and tigers have much higher levels of PEA than herbivores, sometimes 3,000 times as much. American scientists first isolated it from the urine of bobcats (photo, left), which is used by American gardeners to scare off rodents and rabbits. They found that it activates one of the mouse's trace amine-associated receptors (TAAR4), making the mice (and rats) take avoiding action by triggering their aversion circuits. Rodents avoided lion's urine, but not when the phenylethylamine had been removed from it. It may well be that the phenylethylamine levels in carnivores are a product of breakdown of meat proteins, which would be a source of phenylalanine. And it is also possible that Jerry Mouse lacks the important TAAR4 gene.
Tyramine has a structure closely related to phenylamine. You can also call it 4-hydroxyphenethylamine, but its IUPAC name of 4-(2-aminoethyl)phenol, though logical, is perhaps not so intuitive.
Tyramine is formed by the decarboxylation of the amino-acid tyrosine, and is present in significant amount in certain foods, such as some cheeses, leading to the "cheese effect".
 The first monoamine oxidase inhibitor drugs (MAOIs), such as iproniazid, were introduced in the 1950s as antidepressants. They were successful but suffered two side effects; one was liver toxicity, the other as the "cheese effect", which was caused by tyramine. Many foodstuffs such as cheese (but also including other fermented products like draught beer, wine, pizza and yeast products like Marmite) contain significant levels of tyramine, which causes release of noradrenaline. Subsequently high levels of tyramine were found in some common Chinese foods (fermented bean curd, soy sauce and fermented soya bean). The problem was also associated with certain decongestant cold and cough remedies containing molecules like phenylpropanolamine.
The first monoamine oxidase inhibitor drugs (MAOIs), such as iproniazid, were introduced in the 1950s as antidepressants. They were successful but suffered two side effects; one was liver toxicity, the other as the "cheese effect", which was caused by tyramine. Many foodstuffs such as cheese (but also including other fermented products like draught beer, wine, pizza and yeast products like Marmite) contain significant levels of tyramine, which causes release of noradrenaline. Subsequently high levels of tyramine were found in some common Chinese foods (fermented bean curd, soy sauce and fermented soya bean). The problem was also associated with certain decongestant cold and cough remedies containing molecules like phenylpropanolamine.
Normally monoamine oxidase enzymes metabolise tyramine, but the deactivation of the MAO means that noradrenaline levels rise, causing an increase in blood pressure and possibly a fatal hypertensive crisis.
There are two subtypes of MAO enzymes, MAO-A and MAO-B. Both MAO-A and MAO-B metabolise tyramine, but only MAO-B metabolises phenylethylamine. They are found in the intestinal tract, liver and brain, but 80% of intestinal MAO is of the subtype A and is thus mainly responsible for degrading tyramine, so it is inhibition of MAO-A that is mainly responsible for the cheese effect. Subsequently selective MAO-B inhibitors (e.g. selegiline) were developed that do not cause the cheese effect at the correct dose. Nowadays other drugs like tricyclic antidepressants, which act differently, are the drug of choice, and they do not suffer from a cheese reaction. Tyramine and other trace amines have more recently been linked with cluster headaches.
"Aged" cheeses like Stilton can have high tyramine levels, though many cheeses like cottage cheese and the types used on pizzas have only small amounts of tyramine.
![]()
Chapman and Hall Combined Chemical Dictionary compound code numbers: FFC18-E (2-phenylethylamine); BCB62-V (tyramine)
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5255653]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5255653]