![]()
Penicillin
The first antibiotic 'miracle drug'.
![]()
Henry Goss-Custard
Eton College
![]()
Molecule of the Month May 2024
Also available: HTML version.
![]()
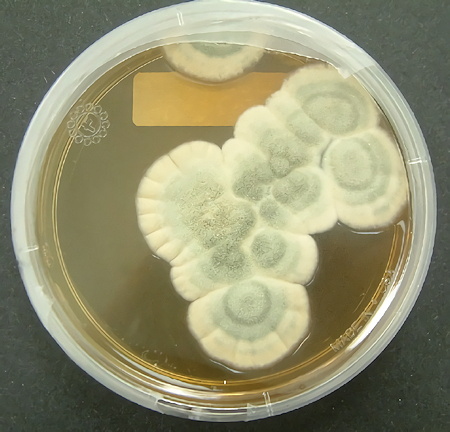
Penicillium mould growing in a petri dish.
[Image: Crulina 98, CC BY-SA 3.0 via Wikimedia Commons]
PenicillinThe first antibiotic 'miracle drug'.
Henry Goss-Custard
Molecule of the Month May 2024
|
 Penicillium mould growing in a petri dish. [Image: Crulina 98, CC BY-SA 3.0 via Wikimedia Commons] |
Penicillin is a naturally-occurring antibiotic used to treat a variety of bacterial infections. It was discovered in 1928 by Alexander Fleming, a Scottish physician and microbiologist working at St. Mary’s Hospital in Paddington, London.
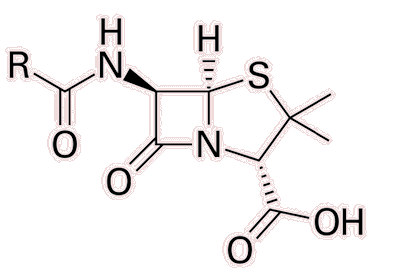 |
|
| The basic structure of penicillin. The R group may vary depending on the different types of penicillin. In Penicillin G, the most common form of peniccillin, R = C6H5-CH2- |
How was it discovered?In the 1920s, Fleming was conducting a series of experimental studies focusing on a widespread type of bacteria known for its 80% fatality rate among the infected: Staphylococcus. Upon his return from holiday on 3 September 1928, Fleming examined his petri dishes containing the Staphylococcus colonies, and noticed something unusual in a dish sat near to an open window. He observed that a blob of mould (soon to be identified as an uncommon strain of Penicillium) was growing in the dish, and furthermore, that the bacteria in close proximity were dying. It appeared that the mould must be secreting a substance that impeded bacterial growth. So what happened next?Despite having made a seemingly groundbreaking discovery, Fleming did not promote it as such, and for good reason: isolating penicillin was an exceedingly challenging task, making the prospect of its development as a drug seem nearly impossible. It wasn't until 1940 that two other scientists, Howard Florey and Ernst Chain, achieved a breakthrough in producing concentrated penicillin from a fungal culture broth, which demonstrated evident bactericidal properties. Soon thereafter, several patients suffering from severe bacterial infections were successfully treated. However, the main challenge at this time remained obtaining sufficient quantities of penicillin. In fact, penicillin was so rare and valuable that it became standard practice to collect urine from patients receiving treatment in order to extract and reuse the penicillin it contained. |
 Alexander Fleming in his laboratory at St Mary's, Paddington, London in 1943. [Image: By Official photographer, TTR 1468 from the Imperial War Museums, Public Domain, via Wikimedia Commons] |
 |
 |
Sir Howard Florey. [Image: Julian Smith, Public domain, via Wikimedia Commons] |
Ernst Chain. [Image: Profgeorgev, CC0, via Wikimedia Commons] |
To solve this problem, Florey, along with several members of his team, endeavoured to persuade the British government to support their vision of mass-producing penicillin, but failed. The US government, however, showed interest, and using deep-tank fermentation (see Fig.6), the mass production and distribution of penicillin was achieved, producing over 600 billion units annually within four years! Fleming, Florey and Chain had revolutionised the fight against bacteria, for which they shared the 1945 Nobel Prize in Medicine.
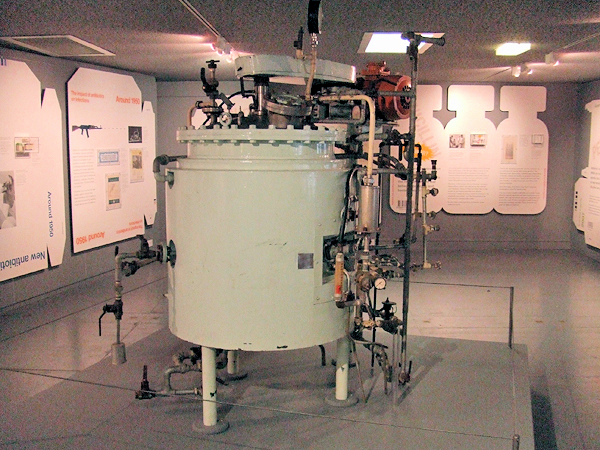
Deep-Tank Fermenter used to grow penicillium.
[Image: Matt Brown, CC BY 2.0 via Wikimedia Commons]
Penicillin can indeed be chemically synthesised, and this was achieved in the 1950s at MIT by John C. Sheehan, but the process is extremely laborious, requiring very precise control of reaction conditions, and is therefore not considered viable for mass production. Instead, mass production continues to take place using deep-tank fermentation of penicillium mould, from which the penicillin is then separated.
Sheehan’s process for chemically synthesising penicillin is as follows:
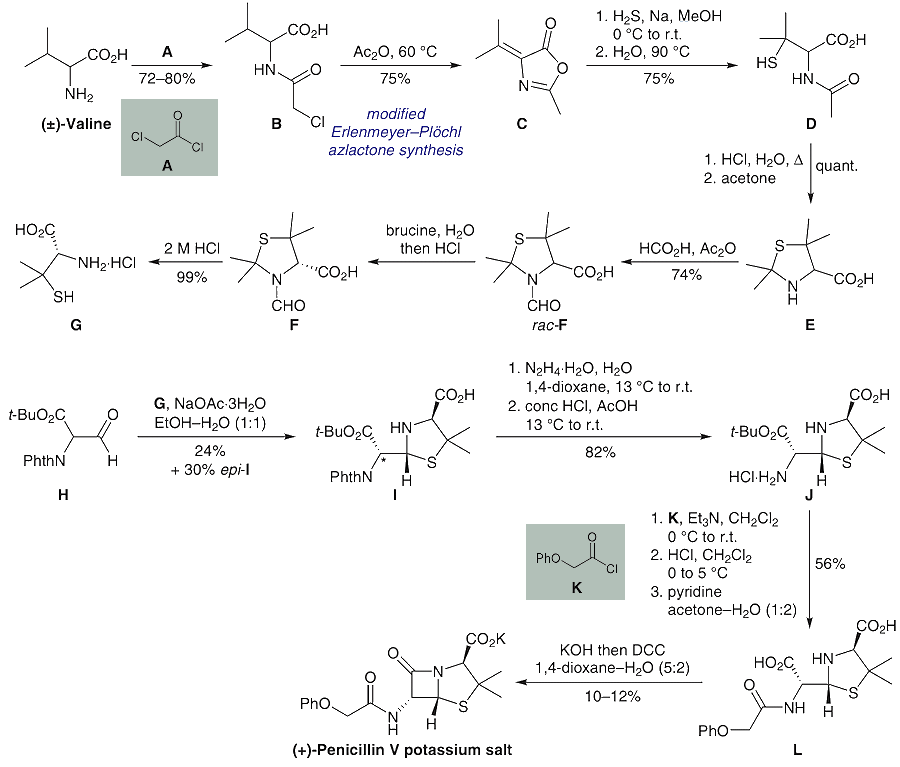
Synthesis of Penicillin as undertaken by J.C Sheehan at MIT in 1959.
[Image: J.C. Sheehan, K.R. Henery-Logan, The Total Synthesis of Penicillin V, J. Am. Chem. Soc. (1957) 79, 1262.]
Well, in short, penicillin works by inhibiting the final stages of something called peptidoglycan synthesis, which bacteria perform when building their cell walls. With a weakened or damaged cell wall, a bacterium cannot survive.
Peptidoglycan. Peptidoglycan is a complicated polymer which forms a rigid wall around a bacterium, providing protection and maintaining its structure. Compositionally, it is made up of two alternating sugar molecules: N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM).
Within the alternating chain of these two sugars, each NAM molecule is also connected to a short peptide chain (a string of amino acids chained together). The rigid nature of peptidoglycan arises from these peptide chains joining one-another in a process known as cross-linking. This results in multiple layers of linked peptidoglycan, and a strong cell wall.

Peptidoglycan
[Image: Bradleyhintze, CC0, via Wikimedia Commons]
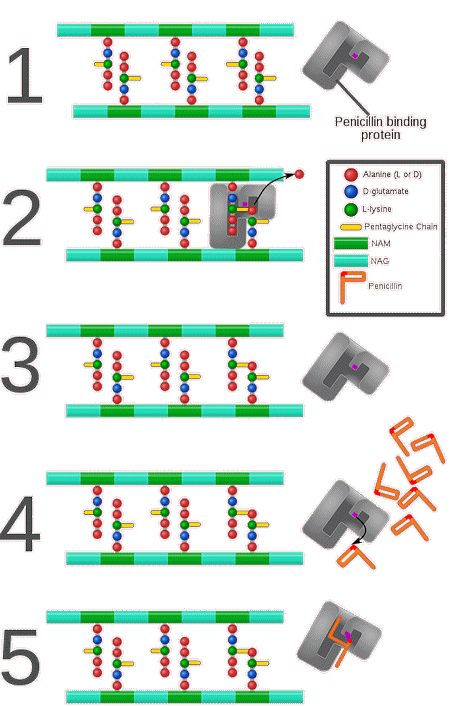
During the cross-linking process, a covalent peptide bond forms between the D-Alanine at the end of one peptide chain and L-Lysine from a chain on an adjacent NAM molecule. This occurs either directly or via a short glycine chain. These cross-links create a strong mesh, allowing the cell wall to withstand the high internal pressures within the cell and to retain its shape. The cross-linking process itself is catalysed by transpeptidase enzymes, which allow the peptide chains to form these covalent peptide bonds. Interestingly, transpeptidase enzymes are often referred to as penicillin-binding proteins (PBPs) because of their affinity for penicillin antibiotics, the importance of which will shortly become clear.
How does penicillin enter the bacterium?
The structure of Gram-positive bacteria offers the molecules of penicillin direct access to the peptidoglycan cell wall making these types of bacteria especially vulnerable to the action of antibiotics. However, the situation with Gram-negative bacteria is a little more complicated, because the lipopolysaccharide acts as a barrier to hydrophilic (water-soluble) substances such as penicillin. Indeed this is the primary purpose of this layer - it functions as an initial protective shield against substances that are toxic to the bacterium. Penicillin can nonetheless enter certain Gram-negative bacteria via small pores in their membrane, known as porins, which exist to allow nutrients to pass into the bacterium, but also allow in antibiotics like penicillin. The rate of entry however is determined by the relative sizes of the penicillin molecule and the porins, meaning that the degree of susceptibility to a specific penicillin varies, although generally speaking, penicillin does not pose nearly as great a risk to Gram-negative bacteria. Once penicillin has penetrated the lipopolysaccharide membrane, the effect it has on Gram-negative bacteria’s thinner peptidoglycan cell wall are much the same. |
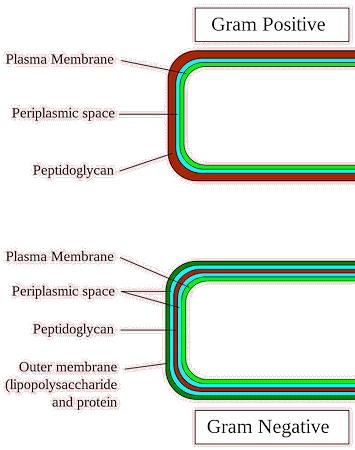 Gram positive and negative bacteria [Image: Graevemoore (talk).Graevemoore at en.wikipedia, CC BY-SA 3.0, via Wikimedia Commons] |
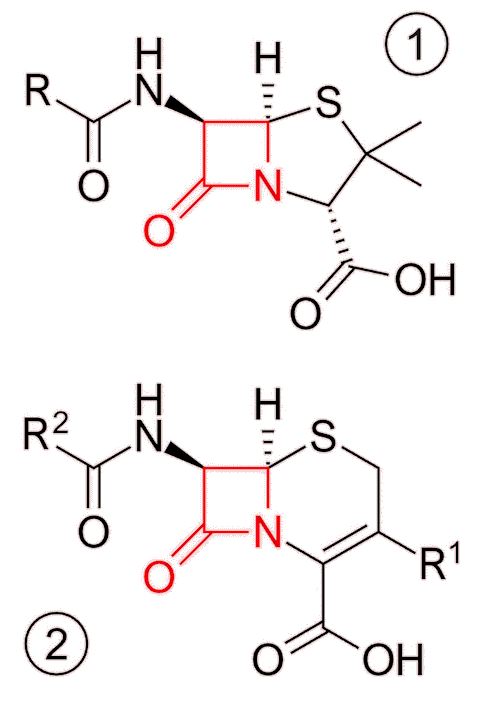
As mentioned above, penicillin works by inhibiting the final stages of peptidoglycan synthesis, therefore weakening the cell wall, and killing the bacteria. Specifically, it is the cross-linking process that penicillin interferes with, blocking the formation of peptide bonds between amino chains. Penicillin, in common with other beta-lactam antibiotics, contains a characteristic structure known as a beta-lactam ring at the molecule’s core which enables it to do this.
 |
 |
| How penicillin (and other beta-lactam antibiotics) inhibit penicillin-binding proteins. [Image: By Mcstrother - Own work, CC BY 3.0, via Wikimedia Commons] |
Skeletal formulae of the basic structures of penicillin (1) and cephalosporin (2) antibiotics, highlighting the beta-lactam ring (red). [Image: Fvasconcellos 19:02, 23 October 2007 (UTC), Public domain, via Wikimedia Commons] |
The beta-lactam ring is a highly reactive four-membered ring, and is a structural analogue to the D-Ala-D-Ala dipeptide found in the peptidoglycan precursor (a molecule that serves as the initial building block for the synthesis of the peptidoglycan layer). Upon entering the active site of this enzyme, this beta-lactam ring mimics the enzyme's natural substrate, tricking the enzyme into initiating its catalytic process: the serine residue in the enzyme's active site attacks the carbonyl carbon of the beta-lactam ring. This attack breaks one of the ring's carbon-nitrogen bonds, leading to the opening of the four-membered beta-lactam ring structure. The opening of the beta-lactam ring alters the structure of the penicillin molecule, enabling it to form a covalent bond with the enzyme, resulting in a new, stable complex. Since the enzyme's active site is permanently modified, it can no longer bind to its natural substrates.
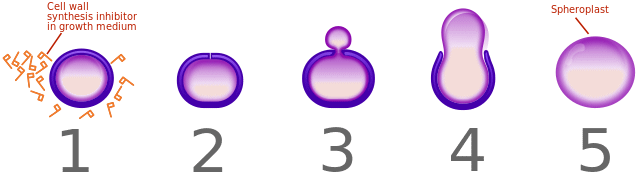
Penicillin’s effects on a bacterium.
[Image: Shudde, CC BY-SA 3.0 via Wikimedia Commons]
The continual prevention of cross-linking results in weakened cell walls with significantly lower structural integrity. This leads to uncontrolled water influx into the bacterium from its surroundings, as it is unable to sustain the proper osmotic balance. This ultimately results in the bursting of the cell and its subsequent death.
In a sense, yes, but remember that whenever bacteria are in a growing phase and undergoing frequent binary fission, cell walls need to be continuously assembled, and during this time they are highly susceptible to interruption by the presence of penicillin.
When a person takes antibiotics to treat a bacterial infection, evolution by natural selection can be massively accelerated. As antibiotics are introduced into a bacterial population, they impose a strong selective pressure on that population. Initially, most of the bacteria in the population are susceptible to the antibiotic, and die. However, a small minority may inherit or acquire genetic mutations that confer a survival advantage, which can cumulatively, and over many generations, give rise to resistant traits within the population.
These bacteria are more likely to survive, reproduce, and transmit their resistant genes to their offspring. With enough time, this process repeats, and eventually, bacterial populations which exhibit almost complete resistance to penicillin (or any other antibiotic for that matter) can emerge, which then go on to spread throughout the world.
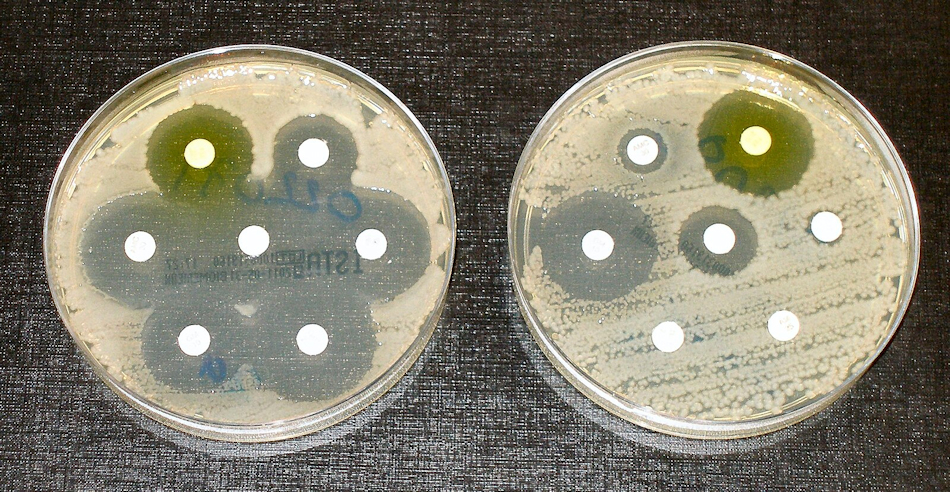
Antibiotic resistance tests.
E. coli bacteria in the culture on the left are sensitive to the antibiotics contained in the white paper discs. The bacteria on the right are resistant to most of the antibiotics.
[Image: Dr Graham Beards at en.wikipedia, CC BY-SA 4.0 via Wikimedia Commons]
These mutations might involve, for example, the enzymatic degradation of the antibiotic, which happens when bacteria start producing an enzyme that can break down penicillin’s beta-lactam ring. Other mutations cause changes to the bacterium’s target proteins that reduce antibiotic binding effectiveness. As is inevitably the case with Darwinian evolution, when bacteria are under sustained selective pressure, nature is extremely ingenious and develops highly sophisticated and innovative techniques to combat the pernicious effect of antibiotics.
Obviously, this is a significant problem for mankind today, and treating an infection caused by a completely resistant bacteria is now very difficult indeed. Before antibiotic resistance was properly understood, antibiotics were used excessively, and with hindsight, recklessly: it was once common practice to apply them to the walls of hospitals with the aim of protecting patients who were particularly susceptible to bacterial infection.
Nowadays, though, there's a concerted effort by medical practitioners to limit antibiotic usage in order to try to preserve their effectiveness. This is why doctors are often reluctant to prescribe antibiotics for minor infections and patients are advised to complete their prescribed antibiotic course (even if symptoms dissipate) to ensure the elimination of all bacteria, and thereby reducing the chance of bacteria developing resistance.
One technique that bacteria have developed to resist antibiotics, and actually the first to be discovered, was the enzymatic destruction of penicillin. Some bacteria developed the ability to synthesise an enzyme, known as penicillinase, and a member of the beta-lactamase family. Beta-lactamases work by hydrolysing the antibiotic’s beta-lactam ring (hydrolysis is a chemical reaction in which water breaks chemical bonds). Without a beta-lactam ring, penicillin will not bind to its target enzyme (transpeptidase), and therefore loses its ability to interfere with cell wall construction. E.coli is a prime example of a bacteria of which almost every strain is highly resistant to penicillin, owing to its production of beta-lactamase enzymes.
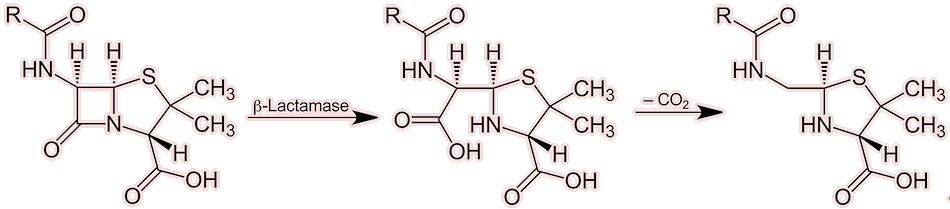
Action of a penicillinase
However, bacterial infections that have developed antibiotic resistance using beta-lactamase enzymes can often still be eliminated with the introduction of beta-lactamase inhibitors. These are drugs which irreversibly bind to beta-lactamase enzymes, allowing the beta-lactam ring in penicillin to remain intact. These drugs are often taken alongside penicillin, when treating a resistant bacteria.
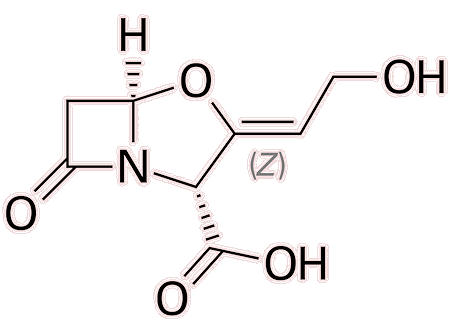 |
|
| Clavulanic acid, a beta-lactamase inhibitor |
The development of antibiotic resistance is a perpetual arms race: as antibiotics are deployed to treat bacterial infections, these bacteria evolve, adopting new mechanisms (such as beta-lactamases) to fight back. This, in turn, challenges us (or nature) to devise more countermeasures, like beta-lactamase inhibitors, to neutralise these defences. This is one reason why biochemistry is so fascinating: evolution ensures that nothing ever stays still, and as a result medicine is always in a race to keep up with evolution.
The penicillin family encompasses a wide variety of antibiotics, each tailored to combat different bacterial infections through slight variations in their chemical structures. These variations primarily involve modifications to the side chain attached to the core beta-lactam ring, which can influence the penicillin’s spectrum of activity (meaning how wide a range of bacteria can the penicillin kill), resistance to degradation by stomach acid, and ability to evade destruction by beta-lactamases, as we have seen.
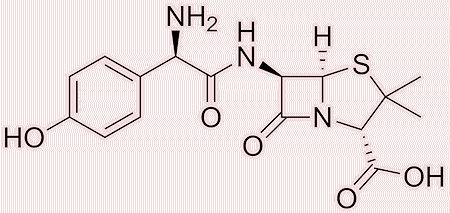 |
 |
| Amoxicillin | Cloxacillin |
While all penicillins operate through the same fundamental mechanism (disrupting the synthesis of the bacterial cell wall by binding to and inactivating penicillin-binding proteins) these structural differences enable some penicillins to be more effective against certain types of bacteria.
For instance, narrow-spectrum penicillins like Penicillin G are highly effective against Gram-positive bacteria, whereas broad-spectrum penicillins, such as amoxicillin, can also target a wider range of Gram-negative bacteria. Some penicillins such as methicillin and oxacillin are able to resist degradation by the bacterial beta-lactamase enzymes produced by some strains of bacteria.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.25579824]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.25579824]