
by
Fabio Pichierri
Molecule of the Month - November 2006
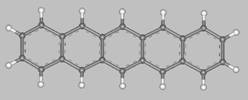
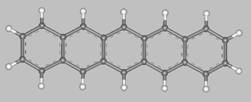
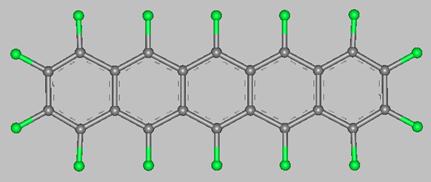
Pentacene (from penta = five, and acenes = polycyclic aromatic hydrocarbons with fused benzene rings)
is a flat-like molecule made of five linearly fused benzene rings, as shown above.
Interest in pentacene has grown dramatically in
recent years as a result of both its crystals and thin films behaving as a
p-type organic semiconductor which can be employed to manufacture electronic
devices such as the organic field-effect
transistor (OFET).
Synthesis
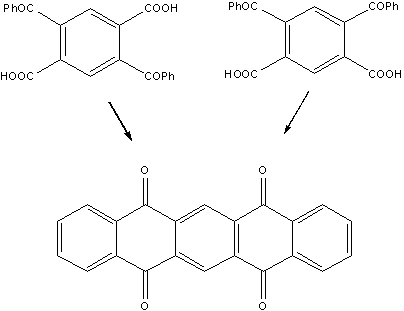
Mills
and Mills of the Northern Polytechnic Institute in
At
this point, pentacene could be obtained simply upon reduction of the diquinone. Furthermore, as stated by
the authors in their paper: "From this diquinone it has been possible to prepare a series of derivatives of dinaphtanthracene".
b,b,b',b'-dinaphtanthracene was the cryptic name used in 1912 to designate pentacene. More efficient synthetic methods
for the preparation of pentacene were devised later on by Marschalk and by Allen and Gates in the 1930s and 1940s. Modern synthetic methods have been devised in order to overcome the low solubility of pentacene in common organic solvents which limits its deposition onto semiconductor surfaces. One of these methods has been recently proposed by a Japanese group of Ehime University (located
in
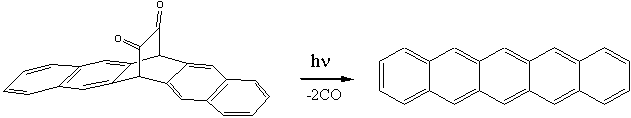
Click for 3D structure of pentacene
Another
important strategy that increases the solubility of pentacene in organic solvents is concerned with the synthesis of pentacene derivatives (see below).
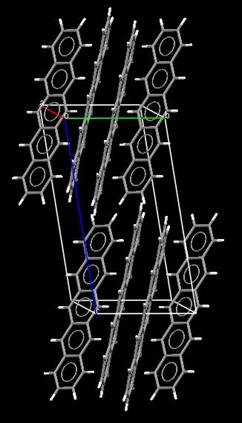
Crystal state In 1961 Campbell and coworkers of the University of Glasgow reported the first
crystal structure determination of pentacene molecular crystals. This experimental study allowed researchers for the first time to accurately determine the molecular dimensions of the pentacene molecule: its length is at ~14 Å and the C-C bond lengths range from 1.381 to 1.464 Å. The peculiar molecular packing adopted by pentacene molecules in the crystal is shown in the figure on the right. The molecules are aligned in parallel
along the a (red) and b (green) crystal axes of the unit
cell thereby forming molecular monolayers in the a,b plane. The monolayers are characterized by d-spacings of 14.1, 14.5 or 15.0 Å depending upon the method of crystallization adopted. Single crystals of pentacene (see picture on the right) can be grown either from organic solvents or by metal-organic vapor-phase epitaxy (MOVPE). The picture on the right (kindly provided by Prof. Takao Someya, head of the Organic Transistor Laboratory, Quantum-Phase Electronics Center, The University of Tokyo) shows a beautiful single crystal of pentacene. |
  |
Electronic
structure
Pentacene possesses 22 p-electrons, one from each carbon atom. It is not surprising, hence, that its frontier orbitals HOMO (highest-occupied molecular orbital) and LUMO (lowest-unoccupied molecular orbital) show strong contributions from the carbon 2pp orbitals, as shown in the following figure (contributions from the carbon 2s atomic orbitals are also present). Obtaining such MO plots is rather straightforward if you have a PC and a versatile molecular modeling software package (in this case I used the semiempirical PM5 method of James J.P. Stewart as implemented in the WinMOPAC software package). The topology of the frontier orbitals HOMO (left) and LUMO (right) of pentacene (see below) agrees well with that obtained from DFT calculations (see the paper by Endres et al.).
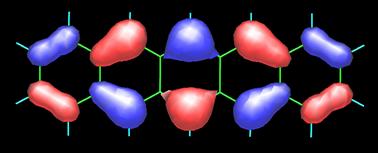
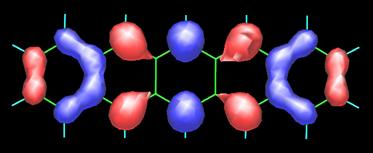
By using quantum chemistry calculations it is also possible to estimate the aromaticity of acenes. This can be done by computing the so called nucleous-independent chemical shifts (NICSs) of each ring centre, as suggested by Paul
von Ragué Schleyer. In this regard, a recent theoretical study of Jemmis and coworkers indicates that the NICSs of pentacene (calculated at the B3LYP/6-311+G** level of theory) correspond to -5.6 ppm (1st ring), -10.8 ppm (2nd ring), and -12.4 ppm (3rd, center ring). In comparison, the NICS of benzene corresponds to -8 ppm.
Pentacene derivatives
A number of interesting pentacene derivatives has been synthesized
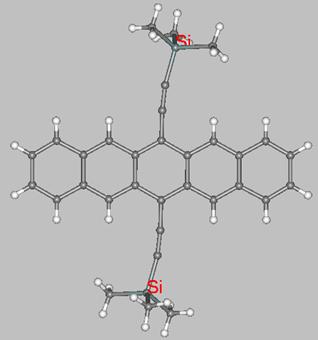
and structurally characterized by X-ray crystallography. For instance, 6,13-disubstituted pentacene derivatives that are highly soluble in organic solvents have been prepared by Anthony and coworkers. Their substituents at positions 6 and 13 are ethyne units (-CºC-) functionalized with SiMe3 groups, as in the 3D structure shown below.
Another interesting derivative is perfluoropentacene which contains fluorine atoms in place of hydrogen. This molecule behaves like an n-type semiconductor while retaining almost the same molecular size as that of pentacene. Theoretical calculations indicate that fully replacing H with F atoms has the effect of decreasing the HOMO-LUMO energy gap from 2.21 eV (pentacene) to 2.02 eV (perfluoropentacene).
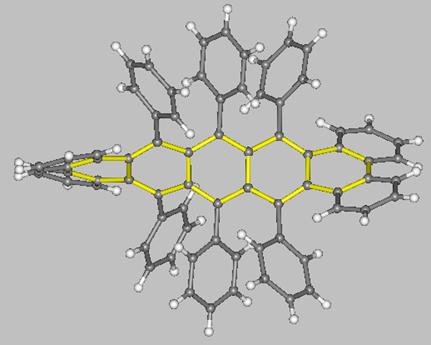
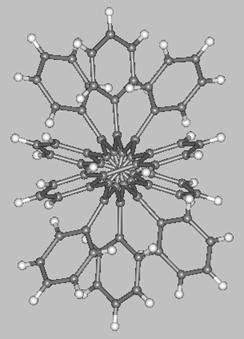
A unique molecule is the pentacene derivative with a 144° twist synthesized by the group of Pascal Jr. (two views of this molecule are shown below). This molecule, which belongs to the D2 symmetry point-group, interconverts to its enantiomer through a C2h-symmetric transition state which, according to MO calculations, is some 12.6 kcal/mol higher in energy with respect to the ground-state enantiomers.
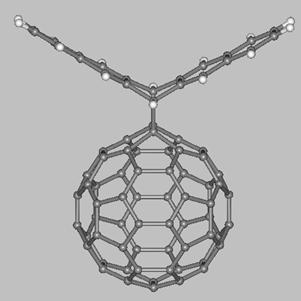
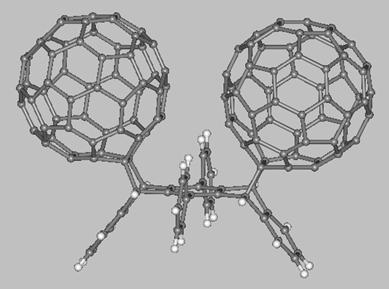
Finally, we mention about two fullerene-acene adducts which have
been synthesized and characterized by Miller, Balch, and coworkers (see drawings below). The first molecule is a C60-pentacene adduct (left side) obtained from a Diels-Alder reaction between [60]fullerene and pentacene while the second molecule (right side) is a bis-[60]fullerene adduct of 6,13-diphenyl-pentacene. The fullerene balls of the latter molecule are present on the same pentacene face which results in the C60 units being at a very close distance (~3 Å). These and others fullerene-acene adducts are expected to display interesting electronic and electrochemical properties which might be exploited in the development of novel functional materials.
Organic electronics with pentacene-based materials
Organic electronics is the field of science and technology that is concerned with the development and use of carbon-based materials in microelectronics. So far, several interesting organic electronic devices have been manufactured using pentacene crystals or thin films. Among them, we mention low-voltage organic thin-film transistors, organic light-emitting diodes, and pentacene
field-effect transistors. These devices are expected to greatly advance the fields of micro- and nano-electronics during the 21st
century. Hence, we can proudly say that molecules are the seeds planted by chemists!
References
Organic Electronics: Materials, Manufacturing, and Applications, H. Klauk (Ed.), John Wiley & Sons, 2006 this book offers a nice overview of the field of Organic Electronics
W.H. Mills, M. Mills, J. Chem. Soc. 101 (1912) 2194 describes the first chemical synthesis of pentacene and some of its derivatives
C.F.H. Allen, J.W. Gates Jr., J. Am. Chem. Soc. 65 (1943) 1502 describes an improved synthesis of pentacene
H. Yamada et al., Chem. Eur. J. 11 (2005) 6212 describes the photochemical synthesis of pentacene and its derivatives
R.G. Endres et al., Comput. Mat. Sci. 29 (2004) 362 presents the results of DFT calculations performed on both pentacene molecule and molecular pentacene solid
R.B. Campbell, J.M. Robertson, J. Trotter, Acta Cryst. 14 (1961) 705 reports the first crystal and molecular structure determination of pentacene
C.C. Mattheus et al., Acta Cryst. C 57 (2001) 939 discusses the polymorphism in pentacene
A.K. Phukan et al., Inorg. Chem. 43 (2004) 5824 discusses the aromaticity of acenes using DFT calculations
J.E. Anthony et al., Org. Lett. 4 (2002) 15 describes the synthesis and crystal structure determination of 6,13-disubstituted pentacenes that are highly soluble in organic solvents
Y. Sakamoto et al., J. Am. Chem. Soc. 126 (2004) 8138 reports the synthesis and characterization of perfluoropentacene
Y. Lu et al., J. Am. Chem. Soc. 126 (2004) 11168 reports the synthesis and structure of twisted pentacene
G.P. Miller et al., Org. Lett. 5 (2003) 4199 discusses about fullerene-acene chemistry
Acknowledgments
I thank Prof. Takao Someya (University of Tokyo) for providing the nice photo of pentacene single-crystal.
◊◊◊◊◊
The crystal structures depicted on this page were retrieved from the Cambridge Structural Database (CSD) maintained by the Cambridge Crystallographic Data Centre (CCDC), 12 Union Road, Cambridge CB2 1EZ, UK
◊◊◊◊◊
I dedicate this MOTM page to my dear brother
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5433379]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5433379]