Over the past few years we have been exploiting the unexplored uses of amine oxides in organic synthesis. In fact, whilst the chemistry of sulfur and phosphine oxides has been studied in detail, the nitrogen analogues have received far less attention. This is suprising when we consider that amine oxides have one of the largest dipole moments of any functional group. As a consequence of this they are invariably hydrated and are often thermally unstable.Work within the group has shown that amine oxides that are intramolecularly hydrogen bonded are stable, highly crystalline materials. Our initial studies focused on the amino acid proline.
(L)-Proline is the only proteogenic amino acid that contains a secondary amino group and as such possesses unique properties in regard to the secondary structure of peptides and proteins. It acts as a helix breaker and unlike the other proteogenic amino acids is capable of forming cis-peptide bonds.

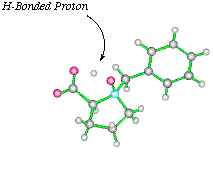
The oxidation of N-benzyl proline with one equivalent of mCPBA yields the highly crystalline N-oxide as a single diasteroisomer. X-Ray analysis of this compound clearly shows the hydrogen bonded proton, actually located closer in space to the N-oxide oxygen. This is also visible in the proton NMR of this compound, the acid proton having a chemical shift of 15ppm.
We believe that hydrogen bonding between the peracid and the carboxylic acid side chain is responsible for the high degree of diasterioselectivity observed in this reaction; the peracid thus delivering the oxygen onto the top face of the proline ring. For example, when N-benzy proline ether ester was reacted with mCPBA at -78oC, a 4:1 mixture of syn:anti N-oxides was produced.
Alcohols are also capable of controlling this diasterioselective oxidation. Thus when N-benzyl prolinol is treated with one equivalent of mCPBA, the corresponding syn N-oxide is produced in excellent yield.
Beta turns are secondary structural motifs commonly found in proteins. We wondered if we could use the very powerful hydrogen ability of the N-oxide to simultaneously interact with two amide groups and, as such, 'lock' the molecule into a particular conformation.
A three dimensional arrangement as in (1) would potentially mimic such a beta turns and allow the development of a novel class of peptidomimetics. This type of molecule is shown below. This highly crystalline material was subject to X-ray analysis.
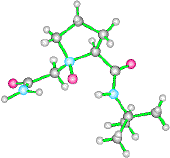
The X-ray shows quite clearly, two hydrogen bonded protons. The overall conformation of the molecule is therefore rigid and locked. NMR analysis of this compound is also quite distinctive, showing two H-bonded protons at low field and one non H-bonded proton.
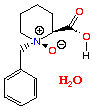
More recently, work has shown that the 6 membered ring analogue can undergo the same selective oxidation. Thus treatment of N-benzyl pipecolic acid with mCPBA, gives rise to the corresponding N-oxide. However, unlike the above examples, the pipecolic acid N-oxide crystallises with one molecule of water, shown above.
A remarkable preference is seen in the pipecolic acid derivatives for formation of axial N-oxides, even without the aid of the directing group. Thus, treatment of N-benzyl tbutylpipecolate with mCPBA at -78oC gave the N-oxide in excellent yield, and in a 25:1 syn:anti ratio! A single recrystallisation yields pure axial N-oxide. 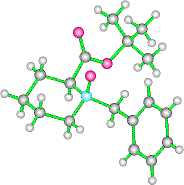
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5469526]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5469526]