![]()
![]()
![]()
Also available: HTML-only, Chime Enhanced, and JMol versions.
![]()
Prostaglandins are important biologically active molecules that resemble hormones in their effects, but are chemically quite different. They received their name because they were first detected in human seminal fluid obtained from the prostate gland. They were not detected for many years because they occur in such low concentrations and have such short half-lives. Prostaglandins are present in almost all cells and tissues, and they are one of the most potent of all biological agents. As little as a nanogram can be biologically active!
Prostanoic Acid - the basic building block of the prostaglandins
In the body, the prostaglandins are based upon the 20 carbon structure of prostanoic acid, which is a fatty acid containing a 5-membered cyclopentane ring. They are named as PG (for prostaglandin), followed by another letter from A-H depending upon the oxidation state and sidegroups of the cyclopentane ring. A subscript usually indicates the number of double bonds in the structure. For example, PGE2 means a carbonyl group at carbon-9 and an -OH group attached to carbon-11, with 2 double bonds in the tail groups. Prostaglandins actually belong to a larger class of compounds called eicosanoids, and a very slight change in their structure or sidegroups can completely alter their biological function.
In general, the effects of the prostaglandins are based on certain broad powers: (a) regulation of the activity of certain smooth muscles, causing them to contract or relax, (b) they induce or prevent secretions from glands, especially endocrine glands, and (c) affect blood clotting and flow. Through these actions they are capable of affecting many aspects of human physiology, and this accounts for their remarkable versatility.
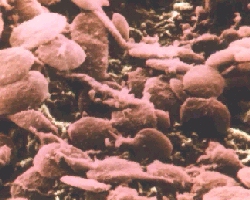
Platelets in blood are activated by prostaglandins and thromboxanes
(such as TXA2) to begin the clotting process.
Prostaglandins can raise or lower blood pressure, and regulate gastric secretions. They are also are responsible for fevers and inflammations, and if these inflammations occur in the blood vessels around the brain, they result in headaches, or the visual effects and pain associated with migraine. Other prostaglandins cause uterine contractions, and so can be deliberately administered to induce labour. Prostaglandins are also used to inhibit the secretion of stomach acids in people with peptic ulcers, treat diabetes and atherosclerosis. Prostaglandins relax certain muscles and are used to relieve asthma and treat high blood pressure.
The prostaglandins are mainly made from arachidonic acid, which itself is synthesised in the liver from linoleic acid found in vegetable oils. An enzyme attaches an oxygen molecule between carbon-9 and 11, to form an endo-peroxide, as well as linking together carbon-8 and 12. Various hydrolysis reactions then turn the peroxide into carbonyl or hydroxyl groups, producing one of a number of primary prostaglandins (e.g. PGE2). This primary compound is the precursor for a whole range of other prostaglandins, which are made by subtly changing the structure or by adding or removing sidegroups.
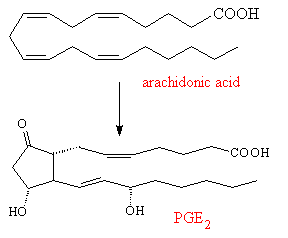 |
When body cells are injured, an enzyme makes prostaglandins, such as PGE2, which creates inflammation, fever and pain. The exact effect depends upon where the damaged cells reside - damage or irritation to skin cells can lead to eczema, to cells in the lungs can initiate asthma, and cells in the blood vessels in the brain can cause headaches. Aspirin acts by blocking the active site on the enzyme, and so prevents synthesis of the prostaglandin PGE2. Thus headaches and other pains or inflammations can be relieved by aspirin. However PGE2 has a second function, it also protects the cells of the stomach wall by inducing the cells to make a protective layer of mucus. Thus aspirin also inhibits this function too, making the cells of the stomach wall more likely to be damaged and less able to repair themselves. Taking too much aspirin, therefore, can lead to stomach problems. The elucidation of the mechanisms by which aspirin works and by which the main prostaglandins are biosynthesised won the 1982 Nobel prize for medicine for John Vane (UK), Sune Bergstrom and Bengt Samuellson (Sweden).
Some other examples of biologically important prostaglandins and their function are:
PGF2α causes contractions in the muscle of the uterus and is used both for abortions and to induce labour. A variation of this, where the hydrogens shown in blue are replaced by CH3 is more stable, and is used for the same purposes under the name Carboprost.
|
PGE1 is a powerful vasodilator, that is, it dilates the blood vessels allowing blood to flow more freely. It is used to prevent gangrene, and in keeping open blood vessels near the heart in cases of angina and stroke. The only difference from PGE2 is the change of the double bond to a single bond between carbon-5 and 6.
PGE1
The action of blood platelets on arachidonic acid gives a number of products, including some fatty acids, plus two new compounds that are called thromboxanes (given the shorthand TX). An example is TXA2 which is a potent muscle contractor and also causes rapid blood clotting. It appears to be important in maintaining a healthy system of blood circulation in the body. The main structural change is an increase in the size of the ring from 5 to 6-members by the insertion of an oxygen atom, and the addition of a peroxide bridge across the ring (although this is rapidly oxidised to 2 -OH groups).
TXA2
Another compound, named prostacyclin (PGI2), has the opposite properties to TXA2 - it relaxes muscles and prevents blood clotting. It is amazing that two similar molecules, made from an identical precursor should have such potent, yet entirely opposite properties! In PGI2 the uppermost tail has been bent around and joined to the -OH group on the ring via an ether bond.
PGI2
The effect of white blood cells on arachidonic acid produces leukotrienes (named after leukocytes). There are a number of these, an example of which is SRS-A (which is actually a mixture of 3 leukotrienes), and they are implicated in the occurrence of asthma. SRS-A is made, along with histamines, when cells in the lungs are damaged or irritated, triggering an allergic response and the contraction of the bronchioles leading to an asthma attack. Various drugs are now being developed which block the cycle of synthesis of these leukotrienes and so prevent the asthma attack. The main difference from the prostaglandins is that the 5-membered ring hasn't formed, and so the compound is still a straight-chained fatty acid. However there is one branching sidegroup, attached via a sulphur-link, and consisting of an amino acid derivative.
LTE4 - one of the 3 leukotrienes comprising SRS-A
![]()