![]()
![]()
![]()
HTML-only version Chime-Enhanced Version VRML version
 Bracken. The curling fern that seems to spreading like a bad, green rash across moor and heath at an alarming rate is not only spoiling the countryside, but harbours a tick that passes lyme disease to humans, causes poisoning in cattle, horses, sheep and people and its spores are one of the latest cancer suspects.
Bracken. The curling fern that seems to spreading like a bad, green rash across moor and heath at an alarming rate is not only spoiling the countryside, but harbours a tick that passes lyme disease to humans, causes poisoning in cattle, horses, sheep and people and its spores are one of the latest cancer suspects.
This botanical pest is considered something of a delicacy in some parts of the world. The Japanese, for instance, often dine on the young bracken sprouts known as fiddleheads (pictured) and it is considered a treat in some areas of Canada and the US too. The trouble is, bracken, while edible, is also highly toxic - especially the fiddleheads - and has been causing bellyache for farmers for centuries where unwary ruminants might graze on the succulent curling shoots.
Bracken poisoning causes depression of bone-marrow activity which leads to severe leukopenia - a form of white blood cell anaemia, - thrombocytopenia - an abnormally low blood platelet count - and hemorrhagic syndrome. In addition, the uncooked plant contains the enzyme thiaminase, which can destroy thiamine (vitamin B1) and cause a possibly fatal disease similar to beri-beri in non-ruminants such as horses.
Not very pleasant. But, in addition to nasty effects on the blood Pteridium aquilinum was also found - in 1960 - to be highly carcinogenic causing polyp-type bladder and intestinal tumours in grazing animals who ate large amounts of bracken or were fed bracken-containing fodder. The carcinogenicity of bracken was demonstrated definitively using lab rats, a result that was later reproduced by several research groups.
It was noted - importantly, from the human consumption perspective - that the young fiddleheads, eaten by the Japanese, are actually the most carcinogenic. Bracken is usually vigorously boiled with wood ash or sodium bicarbonate before it is eaten. "It is eaten alone or as one of the vegetables in soup or as a vegetable mixed with rice," explains chemist Kiyoyuki Yamada of Nagoya University, "cooked bracken is soft and has a good taste that's difficult to explain in English."
Although there is no definitive proof that eating bracken has anything to do with the high incidence of stomach and throat cancer in Japan, knowing a little bit more about what makes the plant so carcinogenic has important implications in studies of cancers in populations that do eat it. But, perhaps more important from the medical point of view is that which ever component of the plant causes cancer it must do so by interfering with DNA in some way. Usually, a molecule that interferes with DNA can itself become an anticancer agent, turning the tables on the cancer cells by damaging their DNA. It could also have potential as an antiviral compound for the same reason.
In 1978, Japanese pathologist Iwao Hirono, formerly of Gifu University and now retired, experimented with aqueous extracts of boiled bracken and found them to be carcinogenic to rats. This indicated to Kiyoyuki Yamada and his colleagues Makoto Ojika, Haruki Niwa and Hideo Kigoshi at Nagoya University that it could be possible to isolate the putative bracken carcinogen in principle. "On the basis of Hirono's result, many researchers around the world attempted to isolate the bracken carcinogen, but they all failed," says Yamada. He and his team found a way.
They began an elaborate research program which culminated in the discovery of the putative agent - ptaquiloside. At first, they could only get a fifth of a gram or so from a kilogram of desiccated bracken powder so studies were hindered somewhat. Once they had developed a better, milder, extraction technique based on efficient solvent-partitioning they could boost this to a gram which was plenty to allow them to accelerate their studies.
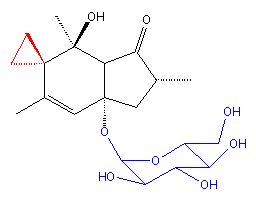 Ptaquiloside |
Ptaquiloside is a colourless and amorphous compound containing a norsesquiterpene with a glucose appendage (shown in blue). The researchers found the compound to degrade quite readily to pterosin B and D-glucose by elimination of D-glucose followed by aromatisation in alkaline solution. Pterosin B turns out to be just one of a group of indanone natural products that had been discovered in bracken and from other sources during the 1970s. The related toxic compounds illudin-S and -M for instance were isolated from the toxic jack-o-lantern mushroom (Omphalotus illudens). Illudins have been evaluated for anticancer activity by US National Cancer Institute scientists so it was logical that the carcinogen ptaquiloside would be evaluated too. The illudins and ptaquiloside have been shown to display selective toxicity for human myelocytic leukaemia and other carcinoma cells.
Ptaquiloside could also be transformed into an unstable dienone in a weak alkaline solution. Yamada and his colleagues found this species to be a powerful alkylating agent for various compounds such as amino acids. In the early 1990s, the researchers set out to determine whether this alkylating ability might be at the root of bracken's carcinogenicity, despite the lack of metabolic activation of ptaquiloside.
They found that the spiro cyclopropyl ring in the dienone (shown in red in the diagram above) opens up and the free end attacks the nitrogen atom in the DNA base adenine. The attached group is then lost to leave a destabilising hydroxyl radical on the group. The result is a DNA chain break. They found after analysing hundreds of DNA models that the AAAT sequence was the most susceptible to attack: this coincided with studies on the cyclopropyl-containing anticancer drug CC-1065. They reasoned that the cytotoxicity of CC-1065 has the same mode of action as the carcinogenicity of ptaquiloside.
The team confirmed the stereochemistry of ptaquiloside from the X-ray structure of a derivative. The next course of action any chemist would wish to take would be to synthesise it. According Yamada and his colleagues writing in Angewandte Chemie recently (1998, 37, 1819-1826): "The instability of ptaquilosin [the sugar-free version of the molecule] poses a multitude of serious problems for its synthesis".
The team, however, devised an approach that adds the loose and angular hydroxy group under very mild conditions in the very last step. They can make the natural enantiomer from off the shelf ingredients including succinic anhydride in 22 steps and convert it to the so-called ultimate carcinogen - the dienone in mild alkali.
The team has now created analogues of the carcinogen, the aim being to find more stable versions that might have improved DNA cleaving powers and be potential anticancer/antiviral agents. They also hope that their research will inspire detailed mechanistic studies into chemical carcinogenesis for bracken.
It is not just human bracken eaters that are at risk: in 1996, researchers reported that ptaquiloside can be passed into milk from cows fed on bracken. Chemist Miguel Alonso-Amelot of the University of the Andes in Venezuela found that almost 9% of the ptaquiloside consumed by the cows emerged in the cows' milk. The research team did not speculate as to the possible effects on human health.
Thinking of a ramble in the countryside...? It might be safer to stick to the sushi bars instead.
![]()
David Bradley is a Science Writer based in Cambridge.
This article first appeared in ChemWeb.com's webzine The Alchemist
![]()
![]() Back to Molecule of the Month page
Back to Molecule of the Month page