What
is Rotenone?
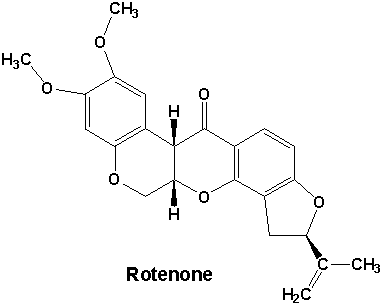
 Rotenone
is a naturally occurring rotenoid plant extract from South America, Australia
and many countries in Southern Asia. It is found in the roots and
stems of several tropical plants, Jewel vine (Derris spp.), Lacepod (Lonchocarpus
spp.) and Hoary Pea (Tephrosia spp.) being the most common.
Rotenone
is a naturally occurring rotenoid plant extract from South America, Australia
and many countries in Southern Asia. It is found in the roots and
stems of several tropical plants, Jewel vine (Derris spp.), Lacepod (Lonchocarpus
spp.) and Hoary Pea (Tephrosia spp.) being the most common.
Rotenone has an empirical
formula of C23H22O6 and has a molecular
weight of 394.41. Its melting point is 165-166°C. Rotenone
is very soluble in many organic solvents, for example alcohol and acetone,
but is almost completely insoluble in water.
Rotenone is generally
unstable and will decompose quickly in water, sometimes as fast as two
weeks after its application. However, it can sometimes persist for
up to six months depending on a variety of factors including light, temperature,
depth, dose and presence of organic debris. Rotenone readily breaks
down in the presence of light into at least 20 products, only one of which, 6ab,
12ab-rotenolone, is toxic. None of the other 
degradation products
are toxic meaning it is considered safe for use on land and in water.
The decomposition process occurs at a faster rate as the temperature of
the water increases. The depth and the presence of organic debris
in the water will affect the amount of light and therefore the rate of
rotenone degradation, as a lack of light corresponds to a slower rate of
degradation.

For centuries, South
and Central American people have used the Jewel vine to stun fish.
When the vine is crushed up and thrown into the water, the fish cannot
inhale oxygen through their gills and come to the surface, making it possible
for them to kill the fish with their bows and arrows. Also, in World
War Two, Rotenone was used to kill lice in the trenches. These are
the two main commercial uses of Rotenone today, as a piscicide and as an
insecticide.
 Rotenone
is a naturally occurring rotenoid plant extract from South America, Australia
and many countries in Southern Asia. It is found in the roots and
stems of several tropical plants, Jewel vine (Derris spp.), Lacepod (Lonchocarpus
spp.) and Hoary Pea (Tephrosia spp.) being the most common.
Rotenone
is a naturally occurring rotenoid plant extract from South America, Australia
and many countries in Southern Asia. It is found in the roots and
stems of several tropical plants, Jewel vine (Derris spp.), Lacepod (Lonchocarpus
spp.) and Hoary Pea (Tephrosia spp.) being the most common.