 |
SilicaThe macromolecule that makes up sand, glass and quartz, and which is a major component of rocks and mountains.
Guillermo Godino Sedano Molecule of the Month - November 2013
|
 |
 |
SilicaThe macromolecule that makes up sand, glass and quartz, and which is a major component of rocks and mountains.
Guillermo Godino Sedano Molecule of the Month - November 2013
|
 |
This article is about silicon dioxide, a molecule which is not really a molecule, at least when it is found in nature, as it forms a giant covalent structure rather than a simple covalent structure. Silicon dioxide or silica is one of the hardest and most common materials in the Earth’s crust. It has a Mohs hardness of 7, being 10 the maximum (diamond). It forms nearly two thirds of the Earth’s continental crust and it is still common further down in the mantle! Even more common are the silicate minerals, 90% of the crust (this includes all minerals which have silica and another element usually one which forms cations, for example magnesium) is made up of these silicates. Pure silica is usually found in the form of quartz (crystalline silica). Finally, its uses are abundant and varied; we will see two of the main ones.
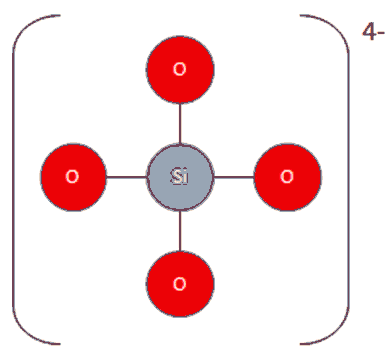
Wait a minute; this is SiO44- not SiO2!
 A peculiarity of the silicon atom makes it preferable for it to bond to four oxygen atoms rather than two double bonds, as SiO2 may suggest. The oxygen atoms form a tetrahedron. Silicon dioxide is not really a molecule; it forms a giant covalent structure with a crystalline arrangement like this (quartz):
A peculiarity of the silicon atom makes it preferable for it to bond to four oxygen atoms rather than two double bonds, as SiO2 may suggest. The oxygen atoms form a tetrahedron. Silicon dioxide is not really a molecule; it forms a giant covalent structure with a crystalline arrangement like this (quartz):

Quartz
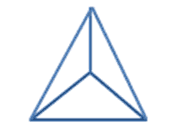 This is quite similar to diamond, although it alternates silicon (grey) with oxygen (red) instead of being all carbon atoms. The silicon atoms bond to the four oxygen atoms in a way which is also similar to carbon in diamond, a tetrahedral (triangular-based pyramid) structure. However, the proportion of silicon to oxygen is actually 1:2, hence the empirical formula SiO2.
This is quite similar to diamond, although it alternates silicon (grey) with oxygen (red) instead of being all carbon atoms. The silicon atoms bond to the four oxygen atoms in a way which is also similar to carbon in diamond, a tetrahedral (triangular-based pyramid) structure. However, the proportion of silicon to oxygen is actually 1:2, hence the empirical formula SiO2.
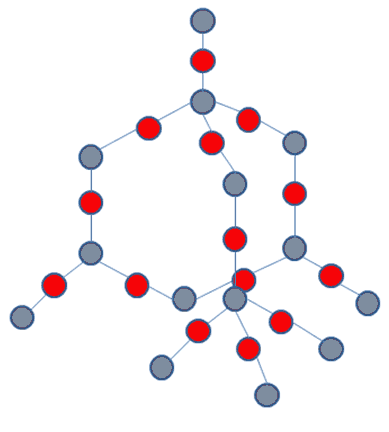
Silicon dioxide forms more than 10 crystalline structures, quartz, tridymite, cristobalite... With other elements it has different structures, for instance faujasite, with a “slightly” more complex formula (Na2, Ca, Mg) 3.5[Al7Si17O48].32(H2O); in this case the other elements are aluminium with sodium, calcium or magnesium, it also has water. These are all three dimensional frameworks, it does not include several other ways to connect the tetrahedrons, such as sheets or chains.
The similarity in structure of quartz and diamond explains its hardness, and quartz is also (in most cases) transparent/translucent, which gives it a use in jewellery.
It may also form an amorphous structure, which we know as glass:
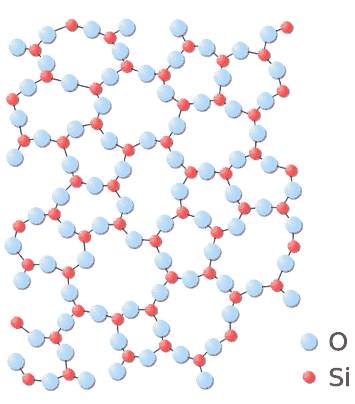
The amorphous structure of glass
 Of course, the most well-known use of glass is in windows. Originally made with pieces of paper, the Romans were the first to use cast glass for windows, however the quality was very low. Since then, glass has become transparent rather than translucent and its use is now common thanks to industrial glass making, which starts with silicon dioxide in sand.
Of course, the most well-known use of glass is in windows. Originally made with pieces of paper, the Romans were the first to use cast glass for windows, however the quality was very low. Since then, glass has become transparent rather than translucent and its use is now common thanks to industrial glass making, which starts with silicon dioxide in sand.
In ‘Lead Glass’, silica (usually from sand) is mixed with potash (K2CO3 and other water-soluble potassium compounds) and red-lead (lead (II, IV) oxide), its use is more recent. Then the mixture is heated up to 1200ºC. The lead oxide is introduced with an air current so that it oxidises completely, since any metallic lead would discolour the glass and attack the fireclay of the furnace. However the lead is useful, since it increases the refractive index (making it shiny) and the elasticity (so it produces a sound when stuck).
When the mixture is prepared one or more (usually more) glass blowers start to use their skill to give the mixture a specific shape and thickness; if the glass is too thick it becomes too heavy, alternatively it may break if it is too thin. Only repeating it once and again will make a glass blower good at his difficult job. The glass is usually blown when inside a mould (called hollowware); however the difference in temperature of more than one thousand degrees will soon decrease (but this is physics, not chemistry). When the glass cools enough, it will become impossible to change its shape, so the glass blowers must be quick and coordinated. Sometimes more glass must be added, to become, for example, a handle; this is done with more molten glass, which will soon ‘fuse’ with the rest of the piece and then cool. If the crystal cools too fast, the widest parts will cool slower and contract less than other zones; this leads to unwanted stress which could break the glass and is prevented by using an annealing oven, which moves the glass through a temperature gradient so the cooling is more homogenous.
The last steps are decoration (a glass cutter draws patterns), smoothing and polishing. The latter is done by using acids, which dissolve the exterior layer of glass. This is the process of making glass.

Decorative items made from glass.
An application of silica which is less known is actually in the object which is produced in greatest quantities, this is the microchip or rather, its main components, transistors. Moore’s law states that the number of transistors in each chip would double approximately every two years, and it has worked during half a century. To give you an idea, the CPU with most transistors in the present has 2.5×109, whereas the Apollo mission used CPUs with less than 15,000 transistors each! That is an enormous difference, taking into account the sizes of the computers.
The first transistors were made of germanium, but they were not very efficient and they required very large rooms for the computers to fit in. In the 1950's silicon replaced germanium, helping the processing speed of transistors, nonetheless, they became very sensitive to dust, which could deposit in the exposed silicon junctions and render them useless. In this same decade, a group of workers founded the Fairchild Semiconductor Corporation. One of them, Jean Hoerni would cause one of the greatest revolutions in the history of computing, he came up with the brilliant idea of adding the oxide to the silicon transistors. Silicon is a semi-conductor, silicon dioxide is an electric insulator; silica separated the silicon junctions from air and other transistors. This not only protected the transistors’ from exposure to air, it also enabled transistors to be used in series, opening the path towards integrated circuits. The process to produce transistors requires thermal oxidation to deposit SiO2 layers only a few atoms thick that forms the 'gate' structure in every transistor.
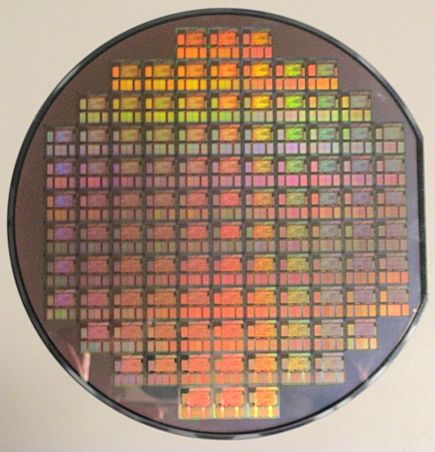
A silicon wafer which will make many Si chips.
This means that you are now using silicon dioxide to read these words. Silicon also gave its name to Silicon Valley. Another substance with very low thickness is graphene, curiously it may now bring a new revolution to computing (from germanium to silicon and then to carbon; all in the same group).
These are only two of the main uses of silica; it is also used to produce optical fibres (fundamental in communications) and aerogel (an extremely light and resistant material), to extract DNA (silica binds to nucleic acids), as an abrasive in toothpaste, to extract its silicon content... It is even important in TV stations and sonars due to its piezoelectric properties (converts mechanical and electrical energy into one another); this is also used in many wrist-watches to measure the flow of time.
As important as these may seem, the favourite use of many people is in a form of silica which is very popular and abundant, sand.

The sand in a sand-castle is made from silica.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5414581]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5414581]