
![]()
(E)-2-butene-1-thiol
And other components of skunk spray
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month February 2022
Also available: HTML version.
![]()
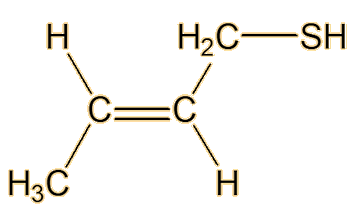
(E)-2-butene-1-thiol
 |
(E)-2-butene-1-thiolAnd other components of skunk spray
Simon Cotton
Molecule of the Month February 2022
|  (E)-2-butene-1-thiol |
Is there anything amusing about skunks?Not unless you like Looney Tunes and Pepé le Pew. Is their name because they smell like cannabis?No. One of the first people to describe their smell said that no sewer ever smelled so bad. Others have said it’s like a combination of rotten eggs, garlic, and burnt rubber. |
 Pepé le Pew - the famous cartoon skunk. [Image: Wikipedia, reproduced according to the US fair-use policy.] |
No, just when they are threatened. First, they give a warning by hissing or stamping their feet. Raising their tail can be followed by spaying a foul-smelling yellow liquid a distance of three metres or more, from two anal glands.
 |
 |
 |
| Striped skunk (Mephitis mephitis), indicating its readiness to spray. [Photo: Wallace Keck, Public domain, via Wikimedia Commons] |
Hog-nosed skunk (Conepatus mesoleucus). [Illustration: Public domain, via Wikimedia Commons] |
Spotted skunk (Spilogale gracilis). [Photo: Public domain, via Wikimedia Commons] |
The first study was reported in 1862, when a German chemist named Swarts found that it involved sulfur-containing molecules. People already knew about very smelly molecules which they called mercaptans (because they ‘captured’ mercury); we now call them thiols. CH3SH, called methanethiol (MOTM May 2017) is possibly the best known.
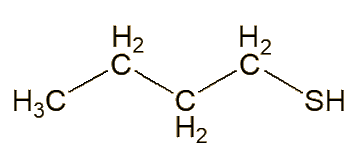
In 1896-7, an American named Thomas Aldrich separated the skunk-spray liquid into two main fractions, one with a boiling range from 100 to 130°C, the other boiling between 130 and 150°C. He noted that butane-1-thiol (aka butyl mercaptan or 1-butanethiol) had a boiling point of 97°C, and suggested that this could be one of the components, based partly on sulfur analyses. Subsequently – for many years - others took this as a statement that this was a key molecule in skunk spray.
 |
|
| Butane-1-thiol |
It was not until 1975 that another two American chemists, Anderson and Bernstein, solved the problem. Compared to the earlier researchers, they had the advantage of physical techniques like 1H NMR and infrared spectroscopy. They found that the spray of the Striped Skunk (Mephitis mephitis) was indeed a mixture, but that its main constituent was not butane-1-thiol but (E)-2-butene-1-thiol. This has a Mr of 88, compared to 90 for butane-1-thiol, so they would both have rather similar sulfur content. They also found that the spray contained very significant amounts of 3-methyl-1-butanethiol.
 |
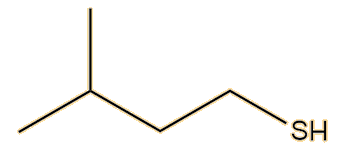 |
| (E)-2-butene-1-thiol | 3-methyl-1-butanethiol |
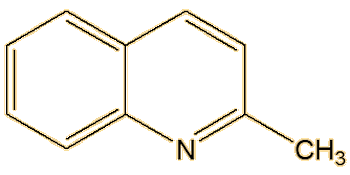
Not at all, there are a number of other components. Back in the 19th century, 2-methylquinoline was identified, and it is the third significant molecule that makes up most of the spray of the striped skunk. Another substance discovered, in much smaller amounts, was later found to be 2-quinolinemethanethiol.
 |
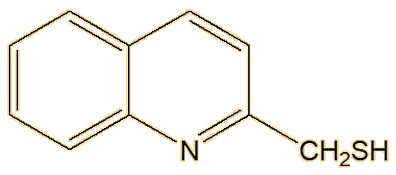 |
| 2-Methylquinoline | 2-Quinolinemethanethiol |
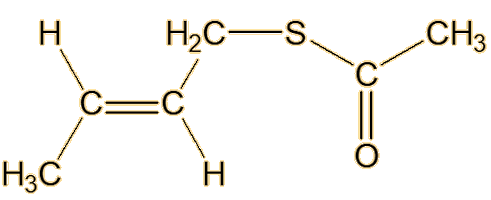
From the 1980s, William F. Wood of Humboldt State University in California, moved skunk-odour research forward again. Looking at the striped skunk, he made a key advance by identifying a new type of component of its spray, in the form of the thioacetates of the already-discovered thiols, such as trans-2-butenyl thioacetate and S-3-methylbutyl-1-thioacetate.
 |
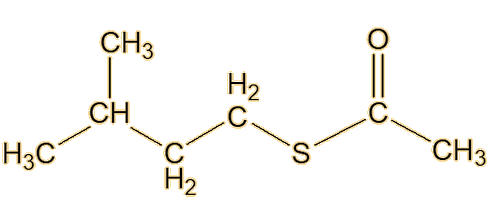 |
| Trans-2-butenyl thioacetate | S-3-methylbutyl-1-thioacetate |
These thioacetates are less smelly than the thiols, but do slowly get hydrolysed by water, releasing the nauseating thiols. This explains why animals (like domestic pets) which were sprayed by a skunk can suddenly start smelling ‘skunky’ again several days later, after getting wet.
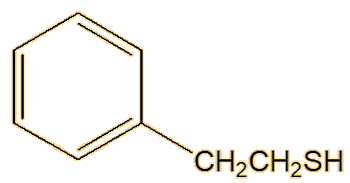
A follow-up study on the spotted skunk (Spilogale gracilis) showed that its spray contained both (E)-2-butene-1-thiol and 3-methyl-1-butanethiol, but not their thioacetates, though it does contain significant amounts of 2-phenylethanethiol.
 |
|
| 2-Phenylethanethiol |
The spray from the hog-nosed skunk (Conepatus mesoleucus) is different again, It produces (E)-2-buten-1-thiol and (E)-2-butenyl thioacetate, but neither 3-methyl-1-butanethiol nor 3-methylbutyl thioacetate, and also no 2-phenylethanethiol.
No one knows for sure. It may be that these related molecules differentiate between different skunk species.
 And how do you get rid of the smell if you (or your pets) get sprayed?
And how do you get rid of the smell if you (or your pets) get sprayed?Some people recommend tomato juice. The best way is to oxidise the thiol to odourless sulfonic acids. This can be done with a mixture of hydrogen peroxide (MOTM Sept 2006) as the oxidising agent, plus baking soda.
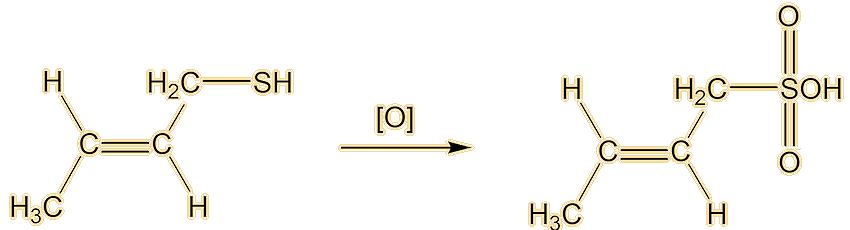
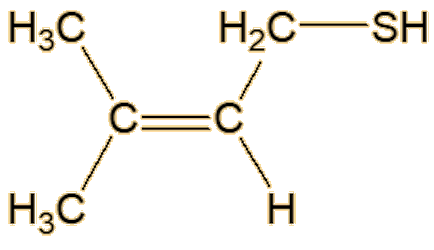
Well, you know how beer is stored in dark bottles. If it is exposed to light it gets an ‘off’ flavour, sometimes called ‘lightstruck’ or even ‘skunking’. Now there’s a clue. Anyway, that is caused by the rather similar 3-methylbut-2-ene-1-thiol. This molecule has also been detected in a Spanish wine.
 |
|
| 3-Methylbut-2-ene-1-thiol |
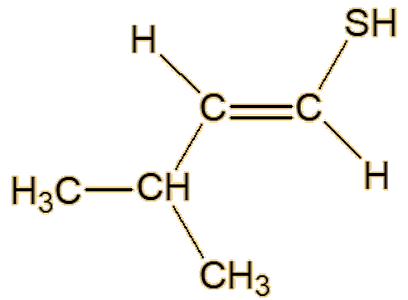
Scientists studying roasted sesame seeds detected a number of unsaturated thiols, including 2-methyl-1-propene-1-thiol, (Z)-3-methyl-1-butene-1-thiol, (E)-3-methyl-1- butene-1-thiol, (Z)-2-methyl-1-butene-1-thiol and (E)-2-methyl-1-butene-1-thiol.
 |
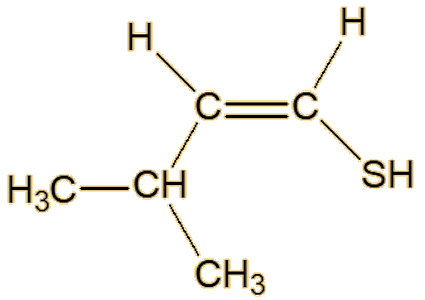 |
| (E)-3-methyl-1-butene-1-thiol | (Z)-3-methyl-1-butene-1-thiol |
D espite cannabis being commonly known as ‘skunk’, just a few weeks before this article was written, the answer to this question would have been ‘no’. The smell associated with different cultivars of Cannabis sativa is very variable, with no sulfur compounds like skunk odorants detected. But very recent analysis (published November 2021) of concentrated extracts of cannabis flowers have detected a number of sulfur-containing odorants, with 3-methyl-2-butene-1-thiol being identified as the primary odorant.
espite cannabis being commonly known as ‘skunk’, just a few weeks before this article was written, the answer to this question would have been ‘no’. The smell associated with different cultivars of Cannabis sativa is very variable, with no sulfur compounds like skunk odorants detected. But very recent analysis (published November 2021) of concentrated extracts of cannabis flowers have detected a number of sulfur-containing odorants, with 3-methyl-2-butene-1-thiol being identified as the primary odorant.
Skunk cabbage is usually associated with parts of North America and with eastern Asia. It has a smell sometimes described as a mixture of skunk, rotten meat and garlic. Recently analysis of odorants escaping from crushed Asian skunk cabbage (Symplocarpus renifolius), photo right, has revealed the presence of a number of sulfur compounds, the two thiols being methanethiol and 1-hexanethiol.
Well, the name of the band was derived from the West-African folk-tales of Anansi, the spider-man of Ghana, with 'Skunk' added to "make the name seem nastier".
One everyday use is that certain saturated thiols, including ethanethiol, 2-propanethiol, 2-butanethiol and t-butylthiol (2-methyl-2-propanethiol), are added to odourless natural gas or bottled gas to give a ready detectable warning of a gas leak.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.17430725]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.17430725]