![]()
 |
Sulphur Dioxide - SO2 |
![]()
Molecule of the Month - December 2003
![]()
Also available: Chime Enhanced, VRML and JMol versions.
![]()
Sulphur forms two main oxides; the gas sulphur dioxide (SO2) and the liquid sulphur trioxide (SO3). Sulphur dioxide is a dense colourless gas, which is soluble in water, and has a suffocating and unpleasant smell of burnt matches. It has a melting point of -72.7°C, and a boiling point of -10°C.
Sulphur dioxide gas can be made directly by heating its constituent elements. Burning molten sulphur in either air or pure oxygen leads to a reaction, which produces a pale blue coloured flame. This looks quite impressive in a darkened room.
S8 (l) + 8 O2 (g) ![]() 8 SO2 (g)
8 SO2 (g)
 Sulphur |
 Burning sulphur to give a blue flame |
 An alternative laboratory preparation is to heat copper turnings with concentrated sulphuric acid (H2SO4), see image, right.
An alternative laboratory preparation is to heat copper turnings with concentrated sulphuric acid (H2SO4), see image, right.
Cu (s) + 2 H2SO4 (aq) ![]() CuSO4 (aq) + SO2 (g) + 2 H2O (l)
CuSO4 (aq) + SO2 (g) + 2 H2O (l)
Sulphur dioxide is an acidic gas and this can easily be demonstrated by adding water and a few drops of universal indicator to a container of the gas. The resulting acid is the weakly dibasic acid sulphurous acid (H2SO3).
SO2 (g) ![]() SO2 (aq)
SO2 (aq)
SO2 (aq) + H2O (l) ![]() H2SO3 (aq)
H2SO3 (aq)
Sulphur dioxide is a major component of acid rain since it mixes with water vapour in the atmosphere, reacting to produce sulphuric acid (H2SO4). This is possible as UV radiation in the upper atmosphere catalyses the reaction between sulphur dioxide and oxygen to produce sulphur trioxide which goes on to react with water. Much has now been done to reduce SO2 emissions with desulphurisation of fuels to help reduce acid rain. Statistics are available on sulphur dioxide levels from 1974 to 1998.
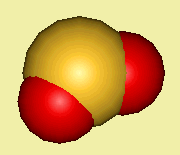
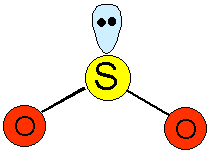 Sulphur dioxide exists as individual covalent V-shaped planar molecules with an OSO bond angle of 120°
Sulphur dioxide exists as individual covalent V-shaped planar molecules with an OSO bond angle of 120°
A simple test for sulphur dioxide is to pass the gas over a piece of filter paper soaked in acidified Na2Cr2O7. The paper goes from an orange colour for the Cr6+ to a green colour for Cr3+. As the oxidation number of chromium is reduced from 6+ to 3+ this clearly indicates a useful property of sulphur dioxide, namely it is a reducing agent. It has found use as an antioxidant, which can help prevent food from spoiling.
| Orange Cr6+ changing to blue-green Cr3+ in the presence of SO2 |
 SO2 is used in fruit drinks to help preserve them. |
 SO2 is used in wines |
The main natural sources of sulphur dioxide are volcanoes, forest fires and oceans. The main human sources of sulphur dioxide are burning fossil fuels, smelting, paper manufacture and the production of sulphuric acid via the Contact Process.
Increasingly there are health concerns with sulphur dioxide as it can trigger asthma in certain individuals. Next time you go to a supermarket why not check food labels to see if they contain sulphur dioxide (additives E220 and E221) or a chemical, which decomposes to produce the gas (E222-8). Sulphur dioxide is found in soft drinks such as fruit juices, some meats and wines. Sulphur dioxide also finds uses as in bleaching and in purifying petroleum products.
Additional ways to produce sulphur dioxide are to drip concentrated sulphuric acid onto a concentrated solution of sodium hydrogen sulphite.
NaHSO3 (aq) + H2SO4 (aq) ![]() NaHSO4 (aq) + H2O (l) + SO2 (g)
NaHSO4 (aq) + H2O (l) + SO2 (g)
GCSE students of Chemistry often come across another way to produce sulphur dioxide in their study of reaction rates in the "disappearing cross" experiment (link slow to load).
Na2S2O3 (aq) + 2 HCl (aq) ![]() NaCl (aq) + H2O (l) + S (s) + SO2 (g)
NaCl (aq) + H2O (l) + S (s) + SO2 (g)
For those who are anxious about the spelling of sulfur click here.
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5437225]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5437225]