BIOCHEMICAL SOLAR CELLS
Example of an electron carrier used in nature. Plastocyanin protein extracted from spinach thylakoid. Electron carrier properties emerge from the association of an organic skeleton (peptide chain) and a metal ion centre (Copper). The tertiary structure is the key function as it maintains the Cu(II) centre in an unfavorable coordination geometry (tetrahedral). This unstability favours quick electron transfer reactions based on Cu(I) / Cu(II) redox couple.
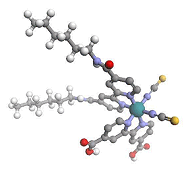
The strategy we used was inspired from plastocyanin proteins. In our dyes we kept the metal ion centre (ruthenium), two surrounding bidentate bipyridines and two monodentate isocyanate ligands. Famous N3 dye contains two 4,4'-dicarboxylic-2,2'-bipyridines. The idea was to keep one of this anchoring bipyridine and a second one able to give hydrogen bondings as we find in proteins. Hydrogen bondings derived from amides bond have for objectives to give:
weak reversible and directed interactions with other surrounding dyes in order to reduce empty space on semiconductor surface. The monolayer load should be increased and by the way light harvesting performance.
an easy chemical group attaching centre. This should permit to attach any desired molecule bearing a primary amine. Dye could be designed to be globally more or less lipophylic or hydrophilic. These two parameters are increasingly important in protein and proved recently to have an influence on electron transfers in semiconductors.
structural active sites with which higher dye molecular ordering on surface could be reached. This was described already in literature with thiol molecules on a gold surface. See example given below.
Scheme synthesis of diverse ligands including the amide LA6 on which different alkyl chains length have been attached.
Red dyes bearing more or less liphophilic groups and amides bonds (C4).
After C4 deposition on TiO2 (from an alcoholic solution), IR measurements demonstrated that addition of 1 or 2 equivalent of urea to the ethanolic dye solution reduces chains disorder. This macro scale modification has a direct sub nanometric impact. The antisymmetric vibration mode in the vicinity of 2900 cm-1 is a diagnostic for the conformational ordering of alkyl chain. Lower values obtained for C1 in comparison to C4 are an indication of a higher conformational ordering of alkyl C6H13 chains. Moreover sharper peaks for C4 indicate higher chain rigidity provided probably by intra-molecular hydrogen bondings. Experiences realised with urea and C4 revealed that conformational chains ordering can be improved as data (table 2) revealed a small decrease of antisymmetric stretching mode frequency from 2930 to 2928 cm-1 and for other C-H frequency from 2961 to 2958 cm-1 and from 2860 to 2858 cm-1.
In conclusion, C4 sensitiser yields incident photon-to-current conversion efficiency values of about 80%. Nevertheless, in addition of good photovoltaic performances of this novel bis amide complex and the possibility to tune self-organisation with hydrogen-bonding recognition groups a new area was opened for the development of supramolecular sciences applied to dye sensitised solar cells.
Bis amide complex C4 [Ru(H2dcbpy)(dhabpy)(NCS)2] presents the best photovoltaïc results with a photocurrent density of 15.5 mA/cm2 and an open circuit potential of 706 mV. A better potential Voc=750 mV and Jsc=14.9 mA/cm2 was obtained with another electrolyte.
The overall conversion efficiency reaches 7.4% under standard AM 1.5 sunlight.
An example of the chemical structure of one such amide N3 dye (Ruthenium (dithiocyanato)(4,4'-dicarboxy-2,2'-bipyridine)(4,4'-dihexylamide-2,2'-bipyridine)) is shown below: