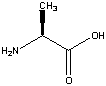
 Glycine
Glycine
The dragline silk of the Golden Orb-Weaving spider is the most studied in scientific research. Spider silk is a natural polypeptide, polymeric protein and is in the scleroprotein group which also encompasses collagen (in ligaments) and keratin (nails and hair). These are all proteins which provide structure. The protein in dragline silk is fibroin (Mr 200,000-300,000) which is a combination of the proteins spidroin 1 and spidroin 2. The exact composition of these proteins depends on factors including species and diet. Fibroin consists of approximately 42% glycine and 25% alanine as the major amino acids. The remaining components are mostly glutamine, serine, leucine, valine, proline, tyrosine and arginine. Spidroin 1 and spidroin 2 differ mainly in their content of proline and tyrosine.
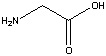
Alanine  Glycine
Glycine

Structure of spidroin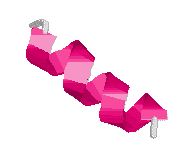
Spidroin contains polyalanine regions where 4 to 9 alanines are linked together in a block. The elasticity of spider silk is due to glycine-rich regions where a sequence of five amino acids are continuously repeated. A 180° turn (b-turn) occurs after each sequence, resulting in a b-spiral. Capture silk, the most elastic kind, contains about 43 repeats on average and is able to extend 2-4 times (>200%) its original length whereas dragline silk only repeats about nine times and is only able to extend about 30% of its original length. There are also glycine-rich repeated segments which consist of three amino acids. These turn after each repeat to give a tight helix and may act as a transitional structure between the polyalanine and spiral regions.
Structure of spider silk
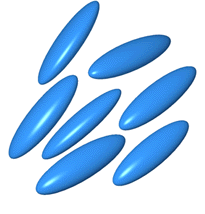 The
fluid dope is a liquid crystalline solution where the protein molecules can move
freely but some order is retained in that the long axis of molecules lie
parallel, resulting in some crystalline properties.
It is thought that the spidroin molecules are coiled in rod-shaped
structures in solution and later uncoil to form silk.
The
fluid dope is a liquid crystalline solution where the protein molecules can move
freely but some order is retained in that the long axis of molecules lie
parallel, resulting in some crystalline properties.
It is thought that the spidroin molecules are coiled in rod-shaped
structures in solution and later uncoil to form silk.
Picture from reference 2
 During
their passage through the narrowing tubes to the spinneret the protein molecules
align and partial crystallisation occurs parallel to the fibre axis.
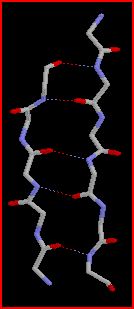
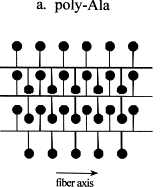
This occurs through self-assembly of the molecules where the polyalanine
regions link together via hydrogen bonds to form pleated b-sheets
(highly ordered crystalline regions).
These b-sheets
act as crosslinks between the protein molecules and imparts high tensile
strength on the silk.
During
their passage through the narrowing tubes to the spinneret the protein molecules
align and partial crystallisation occurs parallel to the fibre axis.
This occurs through self-assembly of the molecules where the polyalanine
regions link together via hydrogen bonds to form pleated b-sheets
(highly ordered crystalline regions).
These b-sheets
act as crosslinks between the protein molecules and imparts high tensile
strength on the silk.
It is not purely coincidence that the major amino acids in spider silk are alanine and glycine. They are the smallest two amino acids and do not contain bulky side groups so are able to pack together tightly, resulting in easier formation of the crystalline regions.
The crystalline regions are very hydrophobic which aids the loss of water during solidification of spider silk. This also explains why the silk is so insoluble - water molecules are unable to penetrate the strongly hydrogen bonded b-sheets.
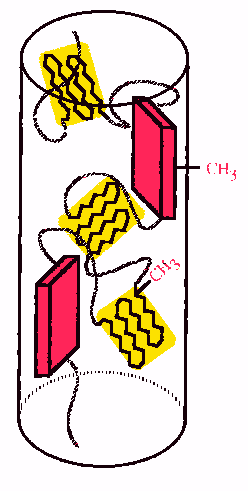 The
glycine-rich spiral regions of spidroin aggregate to form amorphous areas and
these are the elastic regions of spider silk.
Less ordered alanine-rich crystalline regions have also been identified
and these are thought to connect the b-sheets
to the amorphous regions.
Overall, a generalised structure
of spider silk is considered to be crystalline regions in an amorphous matrix.
Kevlar has a similar structure.
The
glycine-rich spiral regions of spidroin aggregate to form amorphous areas and
these are the elastic regions of spider silk.
Less ordered alanine-rich crystalline regions have also been identified
and these are thought to connect the b-sheets
to the amorphous regions.
Overall, a generalised structure
of spider silk is considered to be crystalline regions in an amorphous matrix.
Kevlar has a similar structure.
It is not entirely clear how the protein molecules align and undergo self-assembly to form silk but it may involve mechanical and frictional forces that arise during passage through the spider’s spinning organs.
Image from reference 10
The pink blocks are highly ordered b-sheets and yellow regions are less ordered crystalline areas.