From Hünig's Base to Bis([1,2]dithiolo)-[1,4]thiazines in One Pot: Scorpionine
Charles W. Rees, Carlos F. Marcos, Cecilia Polo, Oleg A. Rakitin, Tomás Torroba, Andrew White and David J. Williams
Department of Chemistry, Imperial College
How can you add structural value, in a one-pot reaction, by mixing two simple commercially available compounds,
breaking 14 and making 12 bonds, in high yield under mild conditions, to produce a new heterocyclic system by an entirely
new method?
Answer:
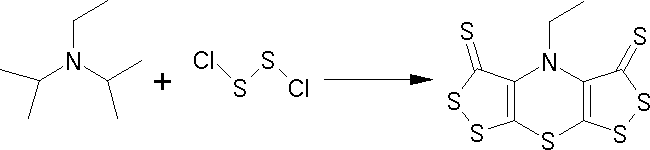
In a complex sequence of about 15 reaction steps, N-ethyl di-isopropylamine (Hünig's base) reacts
with disulfur dichloride (sulfur monochloride) to give the bis([1,2]dithiolo)-[1,4]thiazine (Scorpionine) in an overall yield of 40%.
Remarkable features of this reaction are
- its selectivity (only the iso-propyl groups react, and they react completely, whilst the
ethyl group is entirely inert)
- its overall yield, which requires an average yield of 94% for each of the
15 reactions
- the mild conditions compared to those of other 1,2-dithiole syntheses
- the structure, properties and stability of the black metallic product
The reaction is best performed with an excess of S2Cl2 in 1,2-dichloroethane in the presence
of another (inert!) base such as 1,4-diazabicyclo-[2.2.2]octane (DABCO) for three
days at room temperature, followed by brief heating at reflux. After chromatographic purificaton
the product C8H5NS7, m.p. 202-203°C, is obtained as black
needles with a striking metallic lustre. The symmetrical structure followed from mass spectrometry,
and 1H and 13C NMR, which showed that the N-ethyl group was intact, but that all the 14 C-H bonds of the iso-propyl groups had disappeared, in a striking demonstration of the difference in reactivity of primary and secondary alkyl groups.
If the carbon connectivity of Hünig's base is retained, only a few reasonable
structures are possible for such a stable compound, and spectroscopy indicated the structure
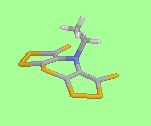
shown for Scorpionine (below), which was confirmed by X-ray crystallography.
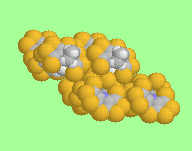 The extended packing of this molecule in the crystal lattice can be see here.
The extended packing of this molecule in the crystal lattice can be see here.
If the reaction is run in the presence of an oxygen source (e.g. formic acid or THF!), two
other products are also formed, with one or both of the thione sulfur atoms replaced by
oxygen. All three products (which have been interconverted) have very
similar shapes and conformations in the crystal lattice. The delocalised dithiole rings are planar, with the fused ring system folded about the thiazine N...S axis, and with the N-ethyl group folder back, scorpion-like, towards the thiazine ring sulphur.
Details can be found in Angew. Chemie, 1997, 36, 281 where a mechanism is
proposed for the reaction. This compound and its analogues are displaying some very interesting
chemistry.

 Back to Molecule of the Month page.
Back to Molecule of the Month page.


 The extended packing of this molecule in the crystal lattice can be see here.
The extended packing of this molecule in the crystal lattice can be see here.