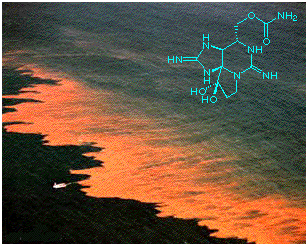

[Molecule of the Month - Septmber 1998]
![]()
![]()
 |
 |
[Molecule of the Month - Septmber 1998]
|
...and the waters that were in the river were turned to blood. And the fish that were in the river died; and the river stank and the Egyptians could not drink of the water of the river...Exodus 7: 20-21
Introduction
In 1987, marine scientists were presented with a series of very disturbing mass die-offs involving humpback whales and other sea-life. In the case of the whales, the number of deaths represented 50 years of natural mortality. The cause of these puzzling deaths was eventually blamed on pollution, since none of the animals showed any signs of disease following post-mortem examination.
By the end of the same year, there had been two more major incidents involving, in these cases, mass poisoning of humans. A whole array of symptoms were reported, including dizziness, diarrhoea and vomiting, disorientation, respiratory distress, and eye irritation. By the end of these poisoning episodes, three people were dead and over a hundred had been severely incapacitated by illness.
 |
 |
When people consume these contaminated shellfish, they very quickly succumb to the effects of the toxin, a syndrone known as paralytic shellfish poisoning (PSP). Death can usually occur within 2-12 hours in untreated cases, and there is no cure. Timely medical assistance, in the form of mechanical artificial respiration is essential, with the result that most human intoxications are non-lethal.
Left: Alexandrium tamarense (c) Larry Fritz |
The algal species itself can grow incredibly fast when conditions are right, leading to spectacular phenomena like that shown above, which are called red tides (or harmful algal blooms). The red tide problem is becoming increasingly prevalent, and has been blamed on polution in coastal areas, and also on farmland run-off into rivers and lakes. Most of the east coast of North America is now affected by harmful algal blooms (HABs) and also a substantial amount of the west coast. The effects of the toxins produced by these HABs have been suffered by people across the world, eg in Australia, and such blooms have resulted in the closure of shellfish farms near the Orkneys for several month each year, subject to results obtained through an extensive monitoring programme.
From Food Poisoning to Chemical Warfare!
The obvious dangers of saxitoxin to public health via contamination throughout the food-chain have been aluded to earlier. A more insidious aspect of the colourful history of saxitoxin has to be its involvement in covert government operations and in chemical warfare. Saxitoxin is about 1000 times more toxic than a typical synthetic nerve gas such as sarin, and it is perhaps not surprising that in the 1950s, the CIA began experimenting with it, reportedly using it in suicide capsules provided to its agents (notably U-2 pilot Francis Gary Powers).
In 1970, President Nixon ordered the CIA to destroy its entire stock of saxitoxin, painstakingly collected over several years, as part of the US commitment in accordance with the United Nations agreement on biological weapons. Nowadays, saxitoxin and ricin are the only two natural toxins classified as Schedule 1 Chemical Warfare Agents. However, in 1975 William Colby, the CIA Director, revealed to Congress that they still possessed over 10 grammes of the material in downtown Washington. Luckily, this supply of saxitoxin was eventually distributed to scientists and medical researchers under the auspices of the National Institutes of Health (NIH).
In connection with this, STX, like many other naturally occurring toxins, has been an indispensible tool in medical research. It is a potent and extremely selective sodium-channel blocker, having no effect on potassium or calcium channels, or the flux of chloride ions, or indeed on acetylcholine release (whereas other marine toxins have similarly selective effects on these aspects of nerve function). Saxitoxin has been used for the labelling, characterisation and isolation of various sodium channel components, and this has opened up new avenues for the study of various nervous disorders.
Chemical Synthesis
The first synthesis of STX was completed by Kishi and co-workers at Harvard in 1977 (J. Am. Chem. Soc. 1977, 99, 2818).
Kishi's synthesis relied on a condensation of a vinylogous carbamate with benzyloxyacetaldehyde and silicon tetraisopropoxide to produce an intermediate thiourea-ester which was converted to a thiourea-urea using standard methods. Cyclisation to provide the saxitoxin skeleton was accomplished readily by treatment with acid, the reaction proceeding via the intermediacy of an iminium ion. Functional group transformations were then carried out to achieve the first total synthesis of racemic saxitoxin.
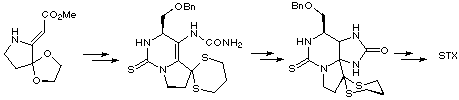
Kishi later published a modified synthesis using chiral auxiliaries to construct (-)-saxitoxin in optically pure form.
The second synthesis was carried out by Jacobi and his collaborators whilst at Wesleyan University, Connecticut (J. Am. Chem. Soc. 1984, 106, 5594).
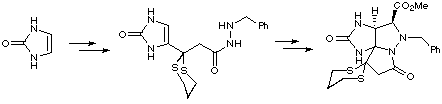
The key step of Jacobi's synthesis of saxitoxin relied on formation of a benzylhydrazide intermediate, which was subjected to methyl glyoxylate hemimethyl acetal and a Lewis acid, in order to construct a highly reactive azomethine imine, which subsequently underwent an intramolecular 1,3-dipolar cycloaddition reaction, leading to an advanced tetracyclic intermediate. This was readily elaborated to accomplish a formal synthesis of the racemic natural product.
Links to other interesting sites
 |
 |
 |
 |
 |
 |
 |
![]()
 |
Last updated 18.8.98 |
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5469421]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5469421]