 |
|
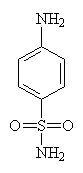
| Sulfanilamide | |
Also available: HTML-only and Chime-Enhanced versions.
When Ernest Fournou of the Pasteur Institute showed that sulfanilamide was the active agent following the breakdown of Prontosil in the body, he opened a whole new chapter in medicinal chemistry. Prontosil is a dye which was produced at the time by German manufacturers I.G. Farbenindustrie and had been shown to inhibit the growth of streptococci in mice. When the company doctor, Gerhard Domagk's daughter contracted an infection her life was saved by an oral dose of Prontosil. Although given as a desperate measure it not only ensured her recovery but Domagk of the Nobel Prize for medicine.
 |
|
| Sulfanilamide | |
As is still the way in medicinal chemistry the discovery of sulfanilamide's efficacy led to searches for similar chemicals - all variations on the structural theme. The aim of the search is typically to reduce the natural toxicity of the parent whilst improving the medicinal effect. Although many thousands of derivatives have been made the most successful have been where the substitution is of one hydrogen on the SO2NH2. For example sulfapyridine was shown to be effective against pneumonia and sulfacetamide was first used in treating urinary tract infections in the 1940's. Sulfathiazole has itself been credited with saving the lives of countless wounded in WWII.
D.D. Woods discovered that sulfanilamide's activity in microorganisms is competitively overcome by para-aminobenzoic acid (PABA) and noted their structural similarity. In these organisms PABA is an essential nutrient in the biosynthesis of folic acid. They die when sulfanilamide is administered because they can no longer make enough folic acid, which is itself important in cell division. The human host is essentially unaffected in this way because folic acid is is obtained from the diet.
Some sulfa- drugs are still in use today but the significance of the story is even greater. As understanding the mode of action led to understanding of the importance of folic acid then studies have continued using folic acid derivatives as potential drugs. One example quoted by T.W. Graham Solomons in his Organic Chemistry text is methotrexate. As a close analog of folic acid it is easily incorporated in some of its biochemcical reactions but inevitably causes the breakdown of these systems. Some of the most important reactions are involved in cell division and methotrexate has been used in treating certain forms of cancer. The drug is toxic to all cells but most sensitive are those which undergo rapid cell division - the cancer cells themselves.
Solomon's Organic Chemistry - 5th Edition, John Wiley & Son Inc.
![]() Back to the Molecule of the Month Page.
Back to the Molecule of the Month Page.