Sulfanilamide (and its relatives)including the drug that saved the life of Winston Churchill
Simon Cotton
Also available: HTML and JMol versions.
|
 |
Sulfanilamide (and its relatives)including the drug that saved the life of Winston Churchill
Simon Cotton
Also available: HTML and JMol versions.
|
 |

The 69-year-old British Prime Minister, Winston Churchill, had two bouts of pneumonia in 1943, the first one in February. At the Teheran conference (photo above), concerned with finalising strategy for the war against Nazi Germany held between November 28th and December 1st that year, he met the President of the United States, Franklin Delano Roosevelt, and Joseph Stalin, General Secretary of the Central Committee of the Communist Party of the Soviet Union.
 After this meeting, he went to General Eisenhower's villa near Carthage but en route spent an hour sitting on an airfield in a cold wind. He slept for the rest of the day, December 11th, then complained of a sore throat. Next day, his temperature rose to 101°F. A portable X-ray machine revealed a shadow on the lung; it was pneumonia again. Immediately Churchill was given the new antibiotic made by the British firm of May and Baker; the tablets were simply known as 'M&B'. Churchill had a mild heart attack on December 15th, but his condition improved and he convalesced by having Pride and Prejudice read to him. He compromised on his habits by agreeing to drink only weak whisky and soda and not to smoke at all. No cigars. He is quoted as saying: "Dear Nurse, pray remember that man cannot live by M & B alone." His doctors, Lord Moran and Dr Bedford, were also M & B.
After this meeting, he went to General Eisenhower's villa near Carthage but en route spent an hour sitting on an airfield in a cold wind. He slept for the rest of the day, December 11th, then complained of a sore throat. Next day, his temperature rose to 101°F. A portable X-ray machine revealed a shadow on the lung; it was pneumonia again. Immediately Churchill was given the new antibiotic made by the British firm of May and Baker; the tablets were simply known as 'M&B'. Churchill had a mild heart attack on December 15th, but his condition improved and he convalesced by having Pride and Prejudice read to him. He compromised on his habits by agreeing to drink only weak whisky and soda and not to smoke at all. No cigars. He is quoted as saying: "Dear Nurse, pray remember that man cannot live by M & B alone." His doctors, Lord Moran and Dr Bedford, were also M & B.
Subsequently Churchill issued a communiqué saying "Excellent nurses and the highest medical authorities in the Mediterranean arrived from all quarters as if by magic. This admirable M & B, from which I did not suffer any inconvenience, was used at the earliest moment and after a week's fever the intruders were repulsed."
At this distance of time, it is not clear whether the drug used was M&B 693, sulfapyridine, or else M&B 760, sulfathiazole, though it is more likely to have been the former. And if it had not been for a German scientist, it is quite possible that this drug might not have been available!
 When World War I broke out in 1914, Gerhard Domagk (1895-1964) was a medical student in Kiel. He immediately joined the German Army and that autumn fought at the First Battle of Ypres. Transferred to the Eastern Front, he was wounded in the leg. After convalescence, he went back to the front as a medical assistant in field hospitals. He saw how easily wounded soldiers contracted the bacterial infection gas gangrene. He swore that he would do all he could to cure these infections.
When World War I broke out in 1914, Gerhard Domagk (1895-1964) was a medical student in Kiel. He immediately joined the German Army and that autumn fought at the First Battle of Ypres. Transferred to the Eastern Front, he was wounded in the leg. After convalescence, he went back to the front as a medical assistant in field hospitals. He saw how easily wounded soldiers contracted the bacterial infection gas gangrene. He swore that he would do all he could to cure these infections.
Even away from the battlefield, people could get fatal infections in all sorts of trivial ways. In 1924, sixteen-year-old Calvin Coolidge Jr., son of the President of the United States, contracted a blister on the toe of his right foot while playing tennis. Septicaemia set in; he was dead in a week. Similarly in November 1930, W.W. ("Dodger") Whysall, a Nottinghamshire and England Test cricketer, slipped on a dance floor, grazed his elbow, and died of septicaemia a fortnight later. After childbirth, women were especially vulnerable to puerperal sepsis ("childbed fever").
After the war, Domagk went back to Kiel and graduated in 1921; he subsequently carried out research and became a university lecturer, before taking the opportunity to direct a laboratory in experimental pathology at I.G. Farbenindustrie, Elberfeld. He was searching for molecules effective against streptococcal infections, and the research concentrated on azo-dyes as chemotherapeutic agents. His team tested hundreds of compounds. After 5 years work, in late 1932, he found that KL730, known later as Prontosil, worked well against streptococcal infections in laboratory mice (in vivo), even at very low doses, though it did not work in test-tube tests against the bacterium (in vitro). I.G. Farben applied for a patent on Christmas Day 1932. Tests on humans with streptococcal infections - for which there was no other treatment - went well, and the drug was patented at the end of 1934 and came into use.
The German patent on KL730 was granted on January 2nd 1935, a few months after a French patent (June 21st). Clinical trials had been carried out in secret and the drug now went on the market; at last Domagk published his first paper on Prontosil in February 1935. Tests were carried out widely in much of Europe in 1935-6, though it was not until well into 1936 before Prontosil reached the USA.
Probably the most important British research on Prontosil was carried out in early 1936 by Leonard Colebrook on behalf of the Therapeutic Trials Committee of the Medical Research Council. Working with Méave Kenny, a colleague at Queen Charlotte's Hospital, he first carried out tests on mice, then on patients suffering from puerperal sepsis. Compared with previous treatments, the mortality rate dropped from over 20% to 4% (see chart below).
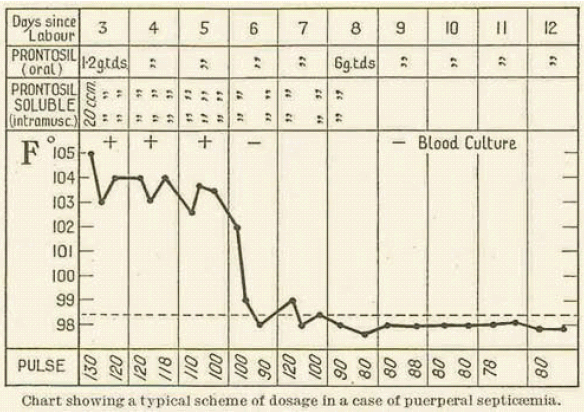
Diagram reference: - L. Colebrook and M. Kenny, The Lancet, (1936), 1319-1322.
On December 4th 1935, 6-year-old Hildegarde Domagk fell on the stairs at home; the needle she was carrying was driven into her hand up to the wrist and broke off. Despite surgical removal of the needle, a streptococcal infection spread through her arm; it looked as if amputation of the arm was her only chance. Gerhard Domagk treated his daughter with Prontosil and the infection cleared up. But the patient who made most headlines was 22-year-old Franklin Roosevelt Jr., son of the President of the United States. He fell ill with a septic sore throat in December 1936 and after injections with Prontosil made a full recovery. This really did bring Prontosil and the sulfa drugs to the public consciousness in the USA.
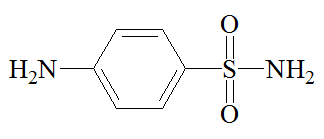
But by then, Prontosil was no longer the last word in antibacterial medications. Over the three days from November 6th to November 8th 1935, Daniel Bovet's team at the Pasteur Institute in Paris discovered that sulfanilamide (4-aminobenzenesulfonamide), was itself active both in vitro and in vivo. Intestinal enzymes in the human body convert Prontosil into sulfanilamide, the active molecule; there were no enzymes in the bacterial cultures upon which Prontosil had been tested, which is why Prontosil was inactive in vitro. One advantage of sulfanilamide over Prontosil was that the skin of patients was not turned red. Moreover, sulfanilamide was much cheaper than Prontosil. It had first been prepared in Vienna in 1908 by Paul Gelmo, in the course of his PhD researches. It was patented in 1909, so that the patent had expired by the time that Bovet's team made their discovery. Bovet was later to receive the 1957 Nobel Prize for Physiology or Medicine.
Sulfanilamide can readily be made from aniline in four steps. Aniline is first acetylated to protect the easily oxidised amine group; sulfonated using chlorosulfonic acid; ammonia is added to generate the sulfonamide group; and finally boiling with dilute HCl removes the protecting acetyl group, which is more easily hydrolysed than the sulfonamide group.
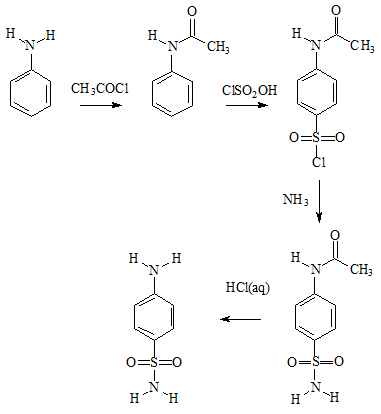
Preparation of sulfanilamide
Sulfanilamide was a great success, but things went badly wrong once in 1937. An American scientist working for a pharmaceutical firm created an "elixir of sulfanilamide," by dissolving the drug in diethylene glycol and adding a dye and raspberry fragrance. This "elixir" was marketed as a treatment for streptococcal infections of the throat. No one had done any animal tests or checked the literature to see if they were using any toxic chemicals. 107 people, mainly children, died as a result.


After this, the elixir was checked to discover the identity of the toxic ingredient. A 23-year-old PhD student, Frances Oldham Kelsey, conducted the animal tests, which showed that it was the diethylene glycol solvent (sweet but very toxic), not the sulfanilamide, that was responsible. She remembered the lessons of this case when, in 1960, she was working for the Food and Drug Administration in Washington, and was asked to evaluate thalidomide.
Public outcry resulted in the 1938 Food, Drug & Cosmetic Act, which created safeguards over the introduction of new drugs - previously a manufacturer did not have to show that its products were safe, but under the Act officials had to review test results and had the power to ask for extra tests to be carried out. Ultimately they had the power to prevent a drug being introduced.
In 1939, the Nobel Committee awarded Domagk the prize for Physiology or Medicine. After the 1936 Peace Prize went to the pacifist Carl von Ossietzky, by then an inmate in a concentration camp (where he was to die 2 years later), Hitler had forbidden Germans to accept Nobel Prizes. Domagk was informed of the award on October 26th 1939, and he sent a guarded acceptance on November 3rd (he did not know if Hitler's ban only applied to the Peace Prize). On November 17th, armed Gestapo surrounded his house and arrested him. Domagk spent over a week in jail and was made to sign a letter turning down the award, which he eventually received in 1947.
The mechanism of sulfanilamide's action was discovered in 1940 by Donald Woods, researching in Oxford. He found that para-aminobenzoic acid (PABA) stopped sulfanilamide's antibacterial effects, and noted that these two molecules had very similar structures.
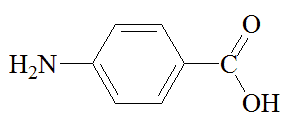
PABA is used by bacteria to make dihydrofolic acid, which is then used to make other molecules including the purine bases necessary to construct DNA, so it is absolutely vital to the bacteria. One stage of the synthesis of dihydrofolic acid is catalysed by the enzyme dihydropteroate synthetase; sulfanilamide fits the active site of this enzyme, and blocks the site to PABA, so that the bacterium cannot multiply and grow. Thus the immune system has the opportunity to clear up the infection.
 |
 |
Sulfanilamide |
p-aminobenzoic acid |
Unlike bacteria, humans do not make dihydrofolic acid. They take in folic acid from their diet as a vitamin (from green vegetables for example), so we don't have any dihydropteroate synthetase, and that's why sulfanilamide is safe for humans.
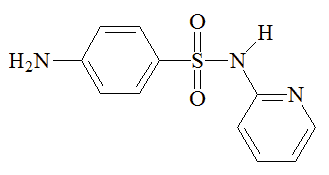
Chemists synthesised other sulfonamides in an effort to find better drugs than sulfanilamide. The best known of these is M&B 693 (sulfapyridine), made at the Dagenham (Essex) laboratories of May and Baker, where a team directed by Arthur Ewins, with chemists including George Newbery and Montague Phillips, were making large numbers of sulfonamides for testing. One day in October 1937, someone spotted a bottle of 2-aminopyridine on a shelf in the lab and decided to make its sulfonamide derivative. What became known as M&B 693 was synthesised on November 2nd 1937. Testing by Lionel Whitby (later Sir Lionel Whitby) at the Middlesex Hospital on pneumococcal and streptococcal mice gave most encouraging results, so human trials came next. In tests carried out by G.M. Evans and Wilfrid Gaisford, at Dudley Road Hospital, Birmingham, 100 patients with lobar pneumonia were treated with M&B 693; the mortality rate was reduced from 78% to 8%. Sulfapyridine went on the market on October 1938, and May and Baker licensed Merck to make it in the USA. It became the preferred treatment for pneumonia, and was credited with saving the lives of 33,000 victims of pneumonia a year in the United States alone.
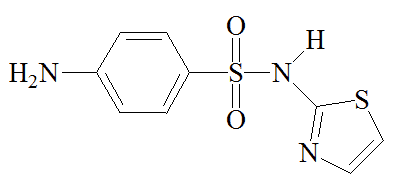
Other drugs such as sulfathiazole (M&B 760) and sulfadiazine followed in its wake. The silver salt of sulfadiazine has been used in burn creams.
 |
 |
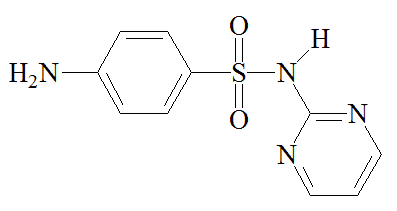 |
Sulfapyridine | Sulfathiazole | Sulfadiazine |
During World War II, each G.I. carried a first-aid pouch containing sulfa powder and a dressing bandage. Whenever anyone was wounded, the sulfa powder was sprinkled into the wound. Medics carried sulfa pills too.

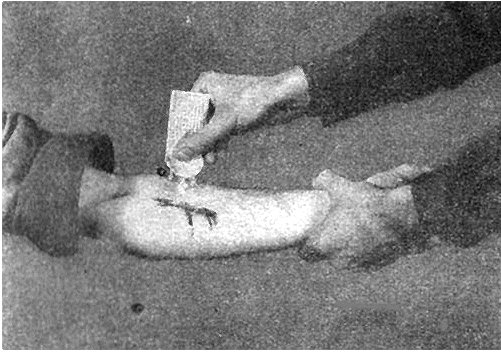
Left: Combat medics in action (Wikimedia Commons). Right: Applying sulfa to an injury (illustration taken from taken from FM 21-11 "First Aid for Soldiers").
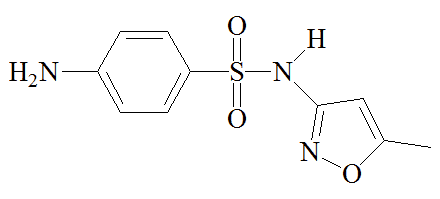
Sulfa drugs are not used as much now as 50 years ago, since some bacteria have acquired immunity to them; there is also the possibility of liver damage and of allergic reactions, such as Stevens-Johnson syndrome. However, other molecules which retain their activity for longer have been synthesized; they tend to be used to treat infections of the urinary tract and in certain sexually transmitted infections like Chlamydia.
A mixture of sulfamethoxazole and trimethoprim, known as Co-trimoxazole, has been used to treat a variety of infections, though this is now more restricted. It has been recommended for HIV-exposed infants and children in certain situations. It can be an effective "broad spectrum" antibiotic against a variety of illnesses because sulfamethoxazole and trimethoprim target successive steps in the biosynthesis of tetrahydrofolic acid, so lower doses of each molecule can be used in the drug. It's a kind of double-whammy treatment, but concerns about side-effects mean that it sees limited use.

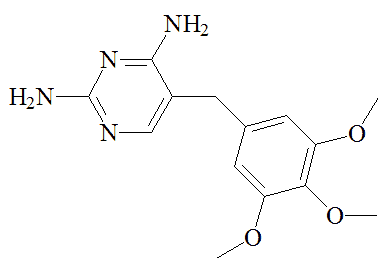
Sulfa drugs were first used in malaria treatment in the 1930s, but were superseded by other agents like chloroquine, which in its turn has become less effective. A combination of sulfadoxime and pyrimethamine (Fansidar) has been used to treat chloroquine-resistant Plasmodium falciparium malaria. This similarly targets the two enzymes in the synthesis of tetrahydrofolic acid. Side effects means that it is only employed for severe cases, and its use is now discontinued in the UK.
 |
 |
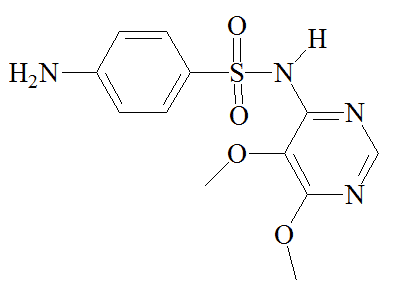 |
Sulfamethoxazole |
Trimethoprim |
Sulfadoxine |
Despite this, sulfonamides were the first widely used and successful examples of chemotherapy, using synthetic chemicals to fight diseases. They were also the first example of the use of a competitive enzyme inhibitor as a drug.
![]()