|
CyanoacrylateSuperglue!
Sarwat Baig
Also available: HTML, JMol, and VRML versions.
|
 |
|
CyanoacrylateSuperglue!
Sarwat Baig
Also available: HTML, JMol, and VRML versions.
|
 |
 History of Cyanoacrylate
History of Cyanoacrylate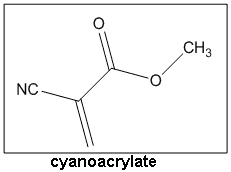 Cyanoacrylate was first invented in 1949 by Dr Harry Coover [1]. At the time Coover was working for Kodak Laboratories where he was trying to synthesise optically clear plastics for precision gunsights during WWII. He was working with cyanoacrylates that seemed to be promising but frustratingly stuck to everything they touched! Coover noted this down but failed to see their potential as adhesives until 1951. At this point, Coover was in charge of a research team who were trying to make a stronger heat resistant acrylate polymer for jet plane canopies. One of his research students made ethyl cyanoacrylate; the main constituent of superglue. On measuring the refractive index to determine the purity of his sample, he found that he could not separate the two prisms and had to show Coover that he had broken an expensive bit of kit! Luckily for both of them, the research student didn't get into trouble as Coover realised that he had a very special adhesive on his hands - something no one had never seen before. Superglue is now a $400 million industry and is now used in many sectors of the chemical and engineering industries.
Cyanoacrylate was first invented in 1949 by Dr Harry Coover [1]. At the time Coover was working for Kodak Laboratories where he was trying to synthesise optically clear plastics for precision gunsights during WWII. He was working with cyanoacrylates that seemed to be promising but frustratingly stuck to everything they touched! Coover noted this down but failed to see their potential as adhesives until 1951. At this point, Coover was in charge of a research team who were trying to make a stronger heat resistant acrylate polymer for jet plane canopies. One of his research students made ethyl cyanoacrylate; the main constituent of superglue. On measuring the refractive index to determine the purity of his sample, he found that he could not separate the two prisms and had to show Coover that he had broken an expensive bit of kit! Luckily for both of them, the research student didn't get into trouble as Coover realised that he had a very special adhesive on his hands - something no one had never seen before. Superglue is now a $400 million industry and is now used in many sectors of the chemical and engineering industries.
The synthesis of cyanoacrylate is based on the Knovenagel Reaction [2]. This is the condensation of formaldehyde (methanal) and an alkyl cyanoacetate. In the first step, an enolate is formed from the alkyl cyanide. The resulting enolate anion acts as a nucelophile and attacks the electrophilic carbon on the formaldehyde. Then the condensation reaction occurs where the -OH group is kicked off, thus forming methyl-2-cyanoacrylate.
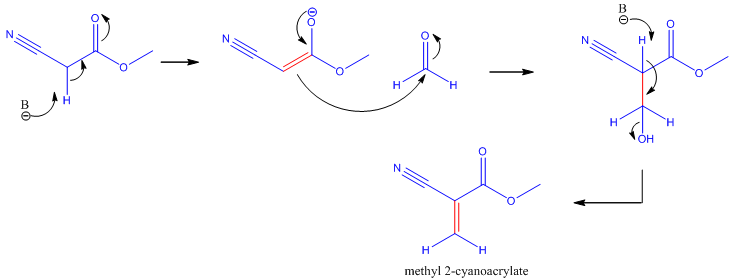
Knovenagel Reaction showing the synthesis of methyl-2-cyanoacrylate. B refers to a base.
When made on an industrial scale [3], the monomer polymerises after the condensation reaction because the monomer is extremely reactive in the presence of base. This means that the polymer needs to be cracked and forms a crude mixture of the monomer and bits of the broken-up polymer. The pure monomer is retrieved by distilling it off from the crude mixture. The rest of the mixture is recycled and cracked again until the all of the pure monomer is retrieved.
In other words, what makes superglue such a strong adhesive? The answer is simple - polymerisation of the cyanoacrylate monomers leads to the formation of extremely strong bonds [4]. The two electron-withdrawing groups (the cyano group and the ester group) make the double-bond extremely vulnerable to nucleophilic attack, and also make the resulting anion extremely stable because the negative charge is pulled across the entire molecule. Hence, cyanoacrylates undergo extremely rapid polymerisation via an anionic mechanism [5], as shown below:
Initiation StepThe nucleophile (Nu-) initiates the reaction [this is frequently a Lewis base (e.g. OH-, amino acids - a lone pair donator)] by attacking the C=C double bond, which breaks and forms a new bond with the nucleophile on one side of the double-bond, and forms the anion on the other side because nothing else adds onto the other side of the double-bond. |
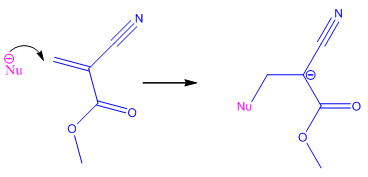 |
Propagation StepThe anion formed in the Initiation Step attacks another cyanoacrylate monomer at the C=C double-bond. The bond formed between the anion generated in the termination step and the new cyanoacrylate monomer is shown in red. This reaction forms another anion, which can then attack another cyanoacrylate monomer. The reaction continues until it is terminated. |
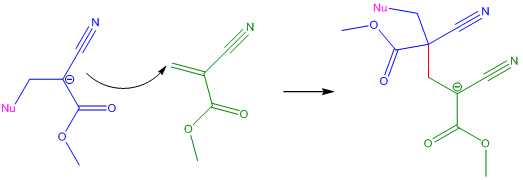 |
The reaction is terminated by the presence of water, air and acidic protons because they react with the nucleophile, effectively quenching the reaction [6]. The type of acid also affects cyanoacrylates; weak acids inhibit and slow down polymerisation whereas strong acids stop polymerisation completely. If the reaction is not terminated the anion exists as a 'living' polymer - this means that should another monomer be introduced into the reaction, the living polymer could propagate further, attack the monomer and lengthen the carbon chain.
Not to worry, you won't be stuck forever! Just find some nail polish remover that contains acetone and the superglue will easily come off. This is because the acetone degrades the polymer and breaks it down, freeing your fingers [7].
The dangers of superglue:
Some unfortunate student fell asleep while his so-called 'mates'
superglued various items to his face....but nothing some acetone can't cure.
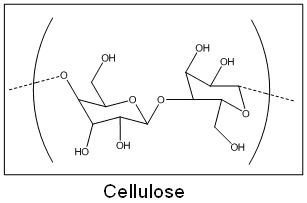
When I was 14, I experimented with some false nails and foolishly knocked over my bottle of false-nail glue over my mum's favourite white cotton hankerchief. Imagine my horror when the hanky started fizzing violently! Cyanoacrylate and cotton react because cotton is made up primarily of cellulose. As you can see from the structure below, cellulose has many hydroxyl (OH-) groups which initiate the polymerisation reaction. Only a trace amount is needed for the reaction to occur. The reaction is extremely exothermic and more often than not, you'll find that the cotton-wool will catch fire! Mixing superglue and cotton is not a good idea - please don't try this at home, even if it is in the name of chemistry.
Coover himself conducted research on the use of cyanoacrylates in medicine whilst working for Johnson and Johnson [1]. He found that cyanoacrylates could be used in place of stitches and sutures [8]. Consequently, medical adhesives have been in use for the past 60 years and were famously used to patch up American soldiers in the Vietnam war. Cyanoacrylates were used as a hemostatic agent which means that they were designed for wounds with unstoppable bleeding (for example chest wounds). The adhesive was administered as a spray, and it was found that a thin layer of the glue was very effective in stopping the bleeding. This ultimately saved hundreds of lives.
I would just like to say now, the superglue you buy in the shops itself is not suitable for use as a medical adhesive [9]. Commercial superglue is made up primarily of short-chain cyanoacrylates such as methylcyanoacrylate or ethylcyanoacrylate which are not compatibile with human tissue. This is because short-chain cyanoacrylates degrade quite quickly and have toxic degredation products which could make the wound even worse! To guard against this, specially made longer-chain cyanoacrylates need to be used for medical applications.
The type of cyanoacrylate used for medical adhesives depend on the part of the body they've been developed for [10]. Opthalmic adhesives use long-chain cyanoacrylates because the longer the chain, the longer it takes for the polymer to degrade and break down. This means there are less breakdown products which irritate the eyes. In tropical countries, medical adhesives are used instead of stitches as this reduces the chance of infection. Medical adhesives are used in cosmetic chemistry as this reduces scarring, used to treat stomach ulcers, lung lesions, soft organ injury as well as being used in dental work to seal tooth sockets after a tooth extraction as this reduced the pain as well as several types of surgery [11].
The idea of delivering medicinal drugs to specific targets in the body via nanospheres (another name for nanoparticles) first emerged in the 1980s [12]. A nanosphere is a hollow spherical cyanoacrylate polymer either filled with the active drug or with the active drug adsorbed on its surface. These nanoparticles deliver peptides, proteins, vaccines or antiproteases to selective targets in the body [13] and can be administered orally or intravenously. One way of loading the nanosphere with the drug is to soak the nanospheres in a solution of the drug which results in surface adsorption. Another way is to use a drug that will electrostatically bind to the anionic polymer then initiate the polymerisation reaction so that the drug is literally trapped inside the nanosphere. Research is still being undertaken to find the method that is the most effective.
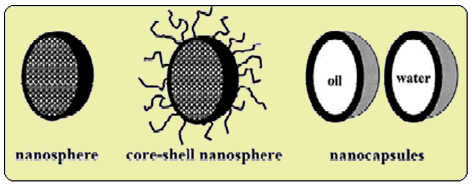
Different types of nanospheres available (from ref.[12]).
Using nanoparticles means that theoretically, the drug is locked up in the particle and isn't used until it reaches its target [12]. Administering intravenously avoid side-effects and chemotherapy resistance. What makes cyanoacrylates particularly suitable for the job is that they bind particularly well to polar substrates - in this case the polar substrates would be human tissue and skin. The initiators in this reaction are the amino acids found in the skin.
There is particular interest in using this for cancer treatment because conventional chemotherapy isn't a pleasant experience for the patient due to the side-effects caused by the chemotherapeutic agents. Side-effects occur because the medicines used eliminate the malignant tumour cells, but along the way they also kill off a lot of the living cells [13].
As the polymers degrade they become increasingly more toxic. However degradation rate and therefore toxicity of cyanoacrylates decrease as the chain length increases. Polyalkylcyanoacrylates are used because they have fast degradation kinetics relative to other polymers used for nanospheres [13]. Cells cope well with cyanoacrylate breakdown products and do not get overloaded with toxins. The rate of release of the drug is also an important aspect. What normally happens is that 70% of the dose is released within 60 minutes of the nanoparticle arriving at the site, with the remaning 30% being released over 120 minutes. Fast release time indicates a poorly encapsulated drug, which could be dangerous for the patient if they receive too high a dose in too short a time. The drugs are normally released faster from nanoparticles made up of short-chain cyanoacrylates owing to their shorter degredation time [13].
This use of cyanoacrylates is also of particular interest as it can be used to let drugs go through the blood-brain barrier [14]. This is very important because the failings of a lot of medicines is their inability to penetrate the blood-brain barrier and enter their site of action. The mechanism for this is currently not known; a good guess is that the particles are able to be taken up by the cells that line the brain capillaries and can get past the blood-brain barrier. This is important for treating ilnesses such as brain cancer although there is a debate over whether to use this method or to create drugs designed to cross the blood-brain barrier [15].
Cyanoacrylate has been used to expose fingerprints since the 1970s [16]. Exposing fingerprints to ethylcyanoacrylate vapour results in a white polymeric layer forming over the ridges [17]. This needs to be done in an enclosed space because oxygen actually inhibits the polymerisation process. This is knows as latent fingerprint fuming. Ethylcyanoacrylate is heated to form a vapour and takes about two minutes to form a polymeric layer over the ridges of the fingerprint. This method is also useful because it can show trace amounts of other exocrine secretions such as blood and sweat, but without destroying either which means that after detecting the blood and sweat, they can be used for DNA testing. Latent fingerprint fuming works best on a non-porous surface such as a metal/plaster and if the material on which the fingerprint is on is white, there are ways of colouring the polymer.

A latent fingerprint brought out using superglue.
Why doesn't it form all over the fingerprint? This is because eccrine sweat (sweat secreted from non-hairy parts of your body, i.e. hands, feet) makes up the ridges on the fingerprint, and therefore there must be something in the eccrine sweat that initiates the anionic polymerisation reaction. Possible initiatiors are amino acids (think of the lone pair on the nitrogen), water (hydroxyl anion) and sodium lactate. The exact mechanism and initiators are currently unknown.
Eccrine sweat becomes contaminated fairly easily, but, the amount of contamination is miniscule in comparison to the other constituents of eccrine sweat that initiate polymerisation. If the fingerprint becomes old and isn't checked for a while, the evidence is not lost. Adding a bit of ammonia encourages polymerisation without damaging the fingerprint.
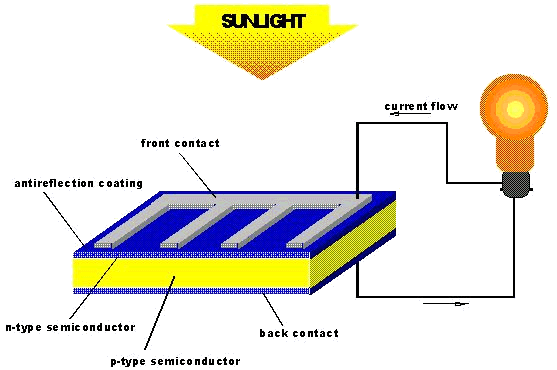 Cyanoacrylate in Solar Cells
Cyanoacrylate in Solar CellsFor a successful solar cell, there needs to be a high solar cell conversion efficiency of up to 10% [18]. Liquid electrolytes have been used for this reason - however, with liquids there is always the problem of the liquid leaking from the solar cell, which poses an enivironmental threat. Solid electrolytes do not have this problem, but they have reduced solar cell conversion efficacy. Cyanoacrylate has been looked at because it can exist as a gel (known as the quasi-solid-state). Gel electrolytes have almost as high a solar cell conversion efficacy as liquid electrolytes but, have a smaller risk of leaking.
Solar cells (or photovoltaic cells) rely on semiconductors - materials whose electrical conductivity increases with increasing temperature [19]. What happens is that an electron from the semiconductor is excited by sunlight (photons) into a higher state. An electric field forces the electron to flow around the cell in one direction until, ultimately it ends up where it began, thus, completing the circuit. In the example shown in the figure below, think of the solar cell in terms of layers. The bottom layer is made up of dye molecules. Photons excite electrons from the dye molecules into the the electrolyte layer. The electrolyte layer is made of cyanoacrylate and tetrapropylammonium cation [N(Pr)4]+ which form a supramolecular complex which conveniently favours anion transfer. This means that it is easier for the electron to go from the excited dye molecules into the next layer - the titanium oxide layer. After the electron goes through the titanium oxide layer, it passes through an external circuit - this generates the current (i.e. flow of electrons). The electrons re-enter the internal circuit through a platinum counter-electrode. The electrons are then transferred back to the excited dye molecules through a redox reaction I3- + 2e ![]() 3I- in which the iodide ions give back the electrons to the dye molecules which would then resume their ground state [18].
3I- in which the iodide ions give back the electrons to the dye molecules which would then resume their ground state [18].
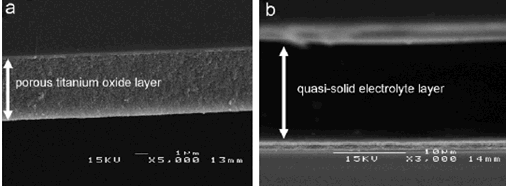
Scanning Electron Microscopy Images of a cross-section of a solar cell,
showing a) the titanium oxide layer and b) the cyanoacrylate quasi-solid layer, from ref.[18].
The supramolecule complex formed between the cyanoacrylate and the tertapropylammonium cation is particular good at carrying anions [18]. The size of the cyanoacrylate monomer means that it is less viscous and it gets through the titanium oxide layer easily; enabling good electron transfer. Cyanoacrylate also has an added advantage in that it displays great mechanical strength and keeps the substrates together.
 How does it work?
How does it work?When photosynthesis takes place the photons excite the electrons into higher states. When the electron is excited into higher states, there are stages where certain reduction reactions take place. The electron is excited via a route called the photosystem II pathway [20]. Cyanoacrylate inhibits the growth of weeds by inhibiting electron excitation which consequently inhibits the photosystem II pathway . The cyanoacrylate herbicides need to be synthesised carefully. For example, they can be made more potent by substituting halogens into the molecule. Substituting a benzyl ring ensures a good fit into the active herbicidal site which would result in greater herbicidal activity. This is a new use for cyanoacrylates and as a result, they are currently not used as herbicides on a commercial scale because large doses are required to have the desired herbicidal effect [21].
Cyanoacrylate is incredibly useful and is found in many areas of the chemical industry. Not bad at all considering it was discovered by accident! However, as Harry Coover once said [1], "One cannot help wondering how many potentially important inventions lie dormant in the recorded observations of scientists which at the time were judged to be irrelevant to their research objective," and concluded wisely, "This should serve as a reminder to all of us to be open minded and curious enough to pursue unexplained events and unexplained results that may unlock new secrets and lead to new and excited discoveries in the future."
![]()