![]()
Tartrazine
The controversial yellow
food-colouring and dye
![]()
![]()
Molecule of the Month May 2018
Also available: JSMol version.
![]()

TartrazineThe controversial yellow
|
 
|
That’s one of those E-numbers isn’t it?Yes, specifically E-102, which is used in EU countries to label food additives. In the US the FFD&C call it ‘Yellow five’ and in other countries it goes by a range of different names. And it is, perhaps, one of the most controversial food additives. How so?Well, tartrazine is a bright yellow pigment that is used in many foods as a colouring. As well as making food and drinks yellow, it can be mixed with other pigments to make a variety of colours, such as green, pink, orange, etc. As such, it is probably the most widely used food colouring in the world. |
 Tartrazine can be purchased as a powder for dyeing. |
The liqueur Galliano uses it for its bright yellow colour. Mushy peas (a UK ‘delicacy’ that often accompanies fish and chips) are bright green as a result of a mixture of tartrazine and another colouring agent (Brilliant Blue FCF, E133). The drink Mountain Dew also contains it, as do many breakfast cereals, such as Life Quaker Oats, processed cheese, pasta, sweets and candies, jams, jellies, mustard, and even bread. It is also found in cosmetics such as lipsticks, toothpaste, mouthwash, shampoos and detergents, and many other common products. Even some medications, such as vitamin pills, throat lozenges, and indigestion tablets may contain tartrazine to give their coatings distinctive, easily identifiable colours.
 |
 |
 |
 |
| Galliano | Mushy peas | Mountain Dew | Life Quaker Oats |
 |
 |
 |
 |
| Lipsticks | Processed cheese | M&M's | Doritos |
 |
 |
 |
 |
| Jellies (Jell-o) | Pasta | Shampoos | Soaps |
A very small percentage of the population (estimated to be less than ~0.1%) appear to be intolerant to tartrazine, and this can reportedly lead them to have symptoms such as itching, hives, coughing, vomiting and even asthma attacks. There have also been links with hyperactivity in children. However, the evidence is still not conclusive - there have not been sufficient proper scientific studies to determine if tartrazine really is the cause of any of these symptoms. Nevertheless, for the 99.9% of the population who are not sensitive to tartrazine the issue remains controversial. Although there is virtually no evidence that tartrazine is harmful to the majority of people, activists still want to ban it ‘just in case’, and tartrazine has become somewhat of a cause célèbre for people who don’t like the idea of their food being full of unnatural ‘chemicals’. The most notorious of the unfounded rumours surrounding tartrazine is that drinking Mountain Dew would have various unwanted effects on a man’s virility, including shrinking the testicles, decreasing the sperm count and/or causing the penis to shrink!
Of course not. It was just the usual internet rumour, nowadays, of course, known as 'fake news'. However, despite the lack of any real evidence that tartrazine is harmful, many countries have nevertheless banned its use in foodstuffs, especially those aimed at children (such as sweets and jellies), although at present it is still legal in the EU and US.
The obvious alternative is beta-carotene [MOTM for April 2002], which is the natural orange pigment found in carrots. However, its colour is not as strong nor as vibrant, which is why manufacturers prefer to use tartrazine if possible. Carotene has the obvious ‘healthy’ credentials of being a natural product, whereas tartrazine is often tainted by originally being made from coal-tar – something that its critics make a great deal of fuss about.
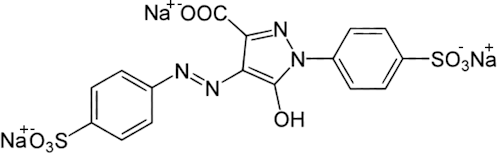
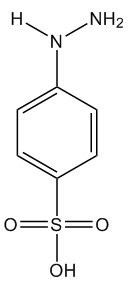
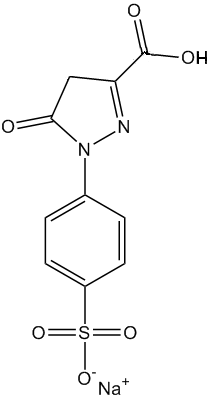
So it’s not made from coal tar now?Originally it was discovered by a German chemist called Johann Heinrich Ziegler in 1884, who was investigating the various new molecules that could be distilled from coal tar for their useful properties. He made a great deal of money from inventing the reaction which produced tartrazine, which at that time was used mostly as a dye for fabrics. Strangely, Ziegler then gave up chemistry, and devoted his life to physics instead, studying fundamental questions about the nature of light, gravity and time. Indeed, he was an influential critic of the new ‘Theory of Relativity’ that an unknown patent clerk called Albert Einstein had just published, and wrote several articles disparaging it. But nowadays, tartrazine is manufactured using a diazo-coupling reaction between sulfonic acid diamine and pyrazolone T. This forms an azo linkage, which is the basis for the high colouration found in all azo dyes. |
 Johann Heinrich Zeigler |
 |
 |
 |

|
| Sulfonic acid diamine | Pyrazolone T | Tartrazine |
Yes, mostly for wool. In fact, it works quite well, being light stable (i.e. it doesn’t bleach with strong sunlight) and washable. The acid groups enable the dye molecule to bind strongly to the amine groups of proteins in the fabric structure. Variations of tartrazine can also be used to dye leather, and as a pigment for artists.
You mean in paintings?Yes, it forms the bright yellow pigment in many paintings, and is mixed with other pigments to make a variety of other colours. Tartrazine replaced the older yellow pigment called ‘Indian yellow’, which was a mixture of hydrated magnesium and/or calcium salts of euxanthic acid, Mg,Ca[C19H15O10]2.nH2O. This ancient pigment was highly prized and expensive, probably because it was purportedly harvested from the urine of cows or camels fed an exclusive diet of mango leaves! Cheap, synthetic tartrazine was an immediate replacement, and became available from about 1890 onwards. Today, this gives art dealers a method of detecting forgeries – any paintings which supposedly dates from before this period cannot contain tartrazine, whereas after that date they almost certainly do (because Indian yellow is virtually unobtainable nowadays except in special museum collections). So a simple non-destructive Raman spectroscopy analysis can easily tell whether a painting dates from before after the discovery of tartrazine – and this has led to the unveiling of many fakes. |
 Indian yellow, in the historical dye collection of the Technical University of Dresden, Germany Photo by Shisha-Tom - CC BY-SA 3.0 |
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5433004]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5433004]