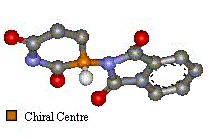
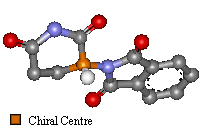
(Click on images for 3D molfiles).

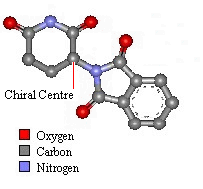
Thalidomide has just one chiral atom and so exists as two enantiomers. The diagram to the right shows the molecule without hydrogens. Notice that two of the groups attached to the chiral centre are part of the same ring structure. They are classified as two different groups. since moving around from the chiral centre the order of atoms is different each way. It is said the chiral atom has two different views around the ring.
 | 
| The only difference is the positioning of the functional groups, yet it was believed this affected much more than the optical activity of the compound. (Click on images for 3D molfiles). |
| 'S' Optical isomer | 'R' Optical Isomer | Reminder of Naming |