Structural Or Constitutional Isomers
Structural Isomers are molecules which have the same molecular
formula but have different connectivities (The Order They Are
Put Together).
Alkanes
can be very simple examples of this.
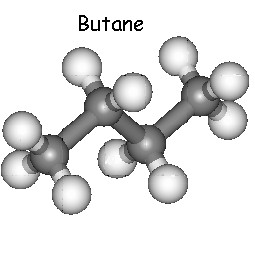
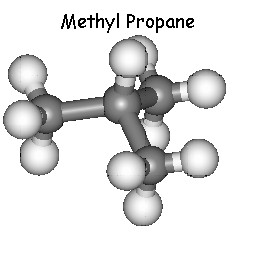
With the structural formula C4H10 there are two
different isomers possible.
As the number of Carbons in an alkane increases, the number of
structural isomers also increases. This happens quite dramatically,
as is shown by the following table.
Number of Isomers of Alkanes
Number of C Atoms | Possible Isomers |
| 1-3 | 1
|
| 4 | 2
|
| 5 | 3
|
| 6 | 5
|
| 7 | 9
|
| 8 | 18
|
| 9 | 35
|
| 10 | 75
|
| 15 | 4,347
|
| 20 | 366,319
|
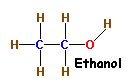
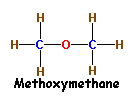
Another simple example of Structural Isomerism is that between
Alcohols and Ethers.
The molecular formula of both Ethanol (An Alcohol) and Methoxymethane
(An Ether) is
C2H6O.
|


|
Alkanes
Alkanes are the simplest class of organic compounds. They contain
only tetravalent (making 4 covalent bonds) Carbon atoms and Hydrogen.
Butane and Methylpropane are two examples which can be found on this
page. As you can find on the other pages of this site they can only
be involved in structural isomerism.