

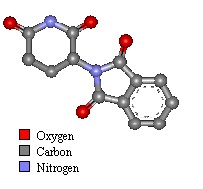
Molecule of the Month - July 2000
Created By Alex Lingham, Undergraduate at Bristol University School of Chemistry. Please send corrections/questions/suggestions to alex_lingham@hotmail.com
For a long time the name Thalidomide has been associated with one of the most horrific medical accidents in history. Despite this, the drug may be set for a come back treating a whole new catalogue of conditions.
This page aims to take a sensitive look at this extraordinary substance which has affected so many lives in so many ways. Looking forward, we see the events which have lead to the possibility of its reintroduction.
THE FIRST APPEARANCE OF THALIDOMIDE
Thalidomide first appeared in Germany on 1st October 1957. It was marketed as a sedative with apparently remarkably few side effects. The Drug Company who developed it believed it was so safe it was suitable for prescribing to pregnant women to help combat morning sickness.
It was quickly being prescribed to thousands of women and spread to most corners of the globe. Nobody had any idea of what was to follow. Drug testing procedures were far more relaxed at this time, and although tests had taken place on thalidomide, they didn't reveal any of its tetragenic (roughly meaning causing malformations) properties. In most countries, drug companies were not required to submit testing results to the appropriate government agencies.
The tests on thalidomide were conducted on rodents which metabolise the drug in a different way to humans. Later tests on rabbits and monkeys produced the same horrific side effects as in humans.
 Towards the end of the fifties, children began to be born with shocking disabilities. It was not immediately obvious what the cause of this was. Probably the most renowned is Pharcomelia, the name given to the flipper-like limbs which appeared on the children of women who took thalidomide. Babies effected by this tragedy were given the name 'Thalidomide Babies'.
Towards the end of the fifties, children began to be born with shocking disabilities. It was not immediately obvious what the cause of this was. Probably the most renowned is Pharcomelia, the name given to the flipper-like limbs which appeared on the children of women who took thalidomide. Babies effected by this tragedy were given the name 'Thalidomide Babies'.
Pictured right are some of babies born with the flipper-like limbs. Remarkably, many of the children involved have gone on to lead successful and fulfilling lives.
SOME EFFECTS OF THALIDOMIDE
Around 12,000 children were born with some kind of disability due to damage caused by thalidomide. Countless more miscarriages, which weren't recorded as caused by thalidomide, mean we can't assess correctly the true scale of the disaster. Estimates put the number of children affected at around 20,000. Even this doesn't take account of the families of the children affected.
One effect of thalidomide is targeting blood vessels. It is able to block growth and so tends to target parts of the body undergoing growth. Pregnant women taking the drug could therefore feel the benefit of the drug in combating morning sickness, but damage was being done to the rapidly growing foetus. It is possible that so many of the thalidomide babies experienced phocomelia because morning sickness can appear around the time of foetal limb growth.
Many other forms of damage were caused to the children, including brain damage. Fortunately many children's disabilities were purely physical and they could learn to cope with disabilities and show normal intelligence.
Not all the effects of this drug were unpleasant, and a positive change has resulted from the disaster. As was mentioned before, at this time it was not usually necessary to submit research to government agencies before approval for sale was given. This was not the case in America.
 The case for Thalidomide was dealt with by reviewing medical officer Frances Kelsey, pictured left. This was her first case and was expected to be a simple approval, with the drug already widely available around the world. Kelsey was not convinced and withheld her approval while more research was conducted. After a while, reports of the drug's tetragenic effects began to emerge from Europe, and the drug was quickly withdrawn the world over. Thanks to her conviction, only 17 thalidomide babies were born in America, mostly due to thalidomide bought in other countries.
The case for Thalidomide was dealt with by reviewing medical officer Frances Kelsey, pictured left. This was her first case and was expected to be a simple approval, with the drug already widely available around the world. Kelsey was not convinced and withheld her approval while more research was conducted. After a while, reports of the drug's tetragenic effects began to emerge from Europe, and the drug was quickly withdrawn the world over. Thanks to her conviction, only 17 thalidomide babies were born in America, mostly due to thalidomide bought in other countries.
Now all countries require this information, and Drug tests have become infinitely more thorough. A lot of research has been put into optically active molecules. In most cases where only one enantiomer of a molecule is an effective drug, a stereo specific manufacturing method will be created.
It was never thought that thalidomide affected the DNA of patients. Nobody expected any effect to be observed in the children of the victims, but unfortunately new research is suggesting effects can be passed through generations.
Of 380 children born to thalidomide victims, 11 have suffered congenital limb defects, a rate 5 times higher than in the general population. Research has suggested it can also alter the DNA of eggs and sperm in rats.
OPTICAL ISOMERISM
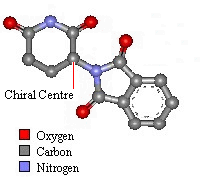
Thalidomide has just one chiral atom and so exists as two enantiomers. The diagram to the right shows the molecule without hydrogens. Notice that two of the groups attached to the chiral centre are part of the same ring structure. They are classified as two different groups. since moving around from the chiral centre the order of atoms is different each way. It is said the chiral atom has two different views around the ring.
Two Images To Show the Enantiomers Of Thalidomide
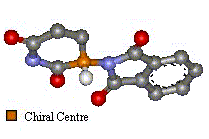 | 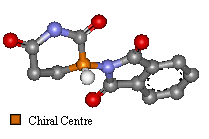 |
The only difference is the positioning of the functional groups, yet it was believed this affected much more than the optical activity of the compound.
(Click on images for 3D molfiles). |
| 'S' Optical isomer | 'R' Optical Isomer | |
The Reality of Optical Isomerism In Thalidomide
Laboratory tests after the thalidomide disaster showed that in some animals the 'S' enantiomer was tetragenic but the 'R' isomer was an effective sedative. It is now known that even when a stereo selective sample of thalidomide (only one of the optical isomers) is created, if administered pH in the body, can cause racemizing. The means that both enantiomers are formed in a roughly equal mix in the blood. So, even if a drug of only the 'R' isomer had been created, the disaster would not have been averted.
The RE-EMERGENCE OF THALIDOMIDE
After the 1960's tragedy and thalidomide being taken out of use, not all the drug was destroyed. A few years later, the story took a surprising turn by means of an Israeli doctor. In 1965 the doctor was treating leprosy patients. One in particular had developed a bacterial infection coupled with inflammation. After stumbling across some thalidomide, remembering it was a sedative, the doctor administered it in the hope it would help the patient sleep and ease his pain.
Remarkably, in the morning the swelling had being brought under control. This extremely fortunate discovery lead to research on the effects of thalidomide on inflammatory diseases.
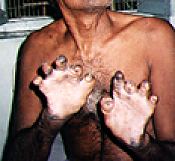 Leprosy is a horrific desease which still causes suffering in parts of the world.
Any new medicines which might help irradicate it are desirable.
Leprosy is a horrific desease which still causes suffering in parts of the world.
Any new medicines which might help irradicate it are desirable.
When our bodies encounter foreign matter cells they become injured and release a protein which increases blood flow. This can cause serious inflamation if the body overreacts. Thalidomide is able to block one of the proteins and stop the body over reacting, surpressing the defences.
This is not the only situation in which Thalidomide has proved effective. Since it reduces growth of blood vessels, it has been tested on cancer patients, as tumours require new blood vessels. It has been used to treat weight loss associated with AIDS/HIV and on Chron's disease patients. It has also been studied for its potential property of stopping HIV progression.
WHAT NOW THALIDOMIDE?
A drug with so many possible benefits would usually be heralded a wonder drug, but the dark side of this compound means some people will never be happy for it to be on the market. Former victims, although afraid to see it re-introduced, don't want to deprive those who could benefit from its positive side of a chance to do so.
It's a difficult situation to risk using this drug. No matter what precautions are taken there is always a high chance more mutated children will be born if it is being used. Some victims have said they would rather it was used properly and legally than banning it and forming a black market. At least then, every effort can be made to educate people and control the drug. They are determined however that the drug should always be called 'THALIDOMIDE' and not sold under a trade name.
The target for all should be to produce drugs with the positive effects of Thalidomide but without the side effects. Celgene Corporation have already produced new therapeutic products structurally related to Thalidomide. Some of these compounds show up to 4000 times the potency of thalidomide and many have lower side effects. Hopefully I'll be able to update this page soon with news of a replacement.

 Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5277799]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5277799]
 Towards the end of the fifties, children began to be born with shocking disabilities. It was not immediately obvious what the cause of this was. Probably the most renowned is Pharcomelia, the name given to the flipper-like limbs which appeared on the children of women who took thalidomide. Babies effected by this tragedy were given the name 'Thalidomide Babies'.
Towards the end of the fifties, children began to be born with shocking disabilities. It was not immediately obvious what the cause of this was. Probably the most renowned is Pharcomelia, the name given to the flipper-like limbs which appeared on the children of women who took thalidomide. Babies effected by this tragedy were given the name 'Thalidomide Babies'.



 The case for Thalidomide was dealt with by reviewing medical officer Frances Kelsey, pictured left. This was her first case and was expected to be a simple approval, with the drug already widely available around the world. Kelsey was not convinced and withheld her approval while more research was conducted. After a while, reports of the drug's tetragenic effects began to emerge from Europe, and the drug was quickly withdrawn the world over. Thanks to her conviction, only 17 thalidomide babies were born in America, mostly due to thalidomide bought in other countries.
The case for Thalidomide was dealt with by reviewing medical officer Frances Kelsey, pictured left. This was her first case and was expected to be a simple approval, with the drug already widely available around the world. Kelsey was not convinced and withheld her approval while more research was conducted. After a while, reports of the drug's tetragenic effects began to emerge from Europe, and the drug was quickly withdrawn the world over. Thanks to her conviction, only 17 thalidomide babies were born in America, mostly due to thalidomide bought in other countries.


 Leprosy is a horrific desease which still causes suffering in parts of the world.
Any new medicines which might help irradicate it are desirable.
Leprosy is a horrific desease which still causes suffering in parts of the world.
Any new medicines which might help irradicate it are desirable.