![]()
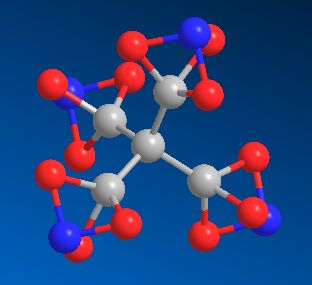
Tetranitratoxycarbon
The explosive molecule discovered by a 10-year-old girl.
![]()
Paul May
University of Bristol
![]()
Molecule of the Month January 2015
Also available: JSMol version.
![]()
|
TetranitratoxycarbonThe explosive molecule discovered by a 10-year-old girl.
Paul May
Molecule of the Month January 2015
|
 |
It happened in a school class in Kansas City, Missouri, USA in 2012. The class were playing with a ball-and-stick molecular-model building kit, and assembling models under the supervision of their science teacher Kenneth Boehr. One pupil, Clara Lazen, assembled a complex model and asked her teacher whether it was a real molecule.

Clara Lazen with her molecule in 2012.
At first the teacher didn't know. The bonding looked ok, and all the atoms had their correct valencies, but to be sure Boehr took a photo of the model on his mobile phone and sent it to a friend, Robert Zoellner, who was a Chemistry Professor at Humboldt State University. Zoellner looked up the structure in Chemical Abstracts and confirmed that although it had the same number and type of atoms as nitroglycerine, this was a unique and previously unrecognized structure.
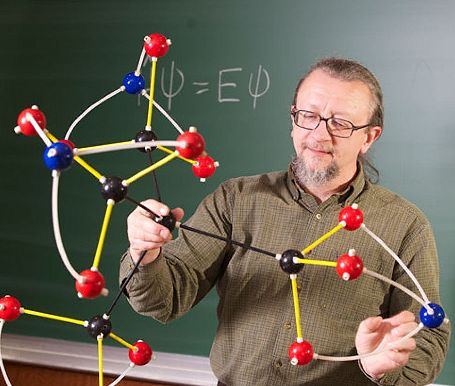
Professor Robert Zoellner admires a model of the new molecule in 2012.
Prof Zoellner made a few calculations to check whether the structure was stable, and then published his findings in a paper in the Journal Computational and Theoretical Chemistry, crediting Lazen and Boehr as co-authors. Boehr says the discovery and publication instigated a new interest in science and chemistry at his school - Clara seemed particularly pleased, although in the last couple of years she's apparently become much more interested in biology and medicine.
Because the molecule contains oxygen, nitrogen and carbon in the same ratios as nitroglycerin, tetranitratoxycarbon has been predicted to be highly explosive. It may also be thermally unstable, but until it's synthesised in a laboratory its properties aren't known. The structure consists of a central carbon surround by 4 other carbons in a standard tetrahedral geometry. But each of these 4 carbons is bonded to 3 oxygens, which in turn are all bonded to a single nitrogen.
![]()
[Thanks to Masoud Sadeghi for suggesting TNOC as a MOTM]
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5259739]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5259739]