![]()
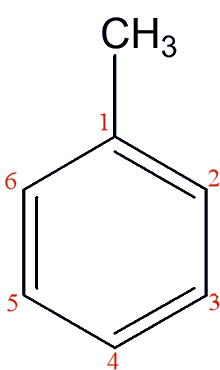
Toluene
a.k.a. methylbenzene
The precursor to TNT.
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month July 2022
Also available: JSMol version.
![]()
|
Toluenea.k.a. methylbenzeneThe precursor to TNT.
Simon Cotton
Molecule of the Month July 2022
|
 |
We are lucky. The first person to identify it (1837) named it rétinnaphte!
You mean, its homologue?

They are both colourless liquids at room temperature. Toluene melts at -95°C and boils at 111°C, thus, its liquid range is 206°, whereas benzene (MOTM Aug 2011) melts at 6°C and boils at 80°C, so its liquid range is just 74°.
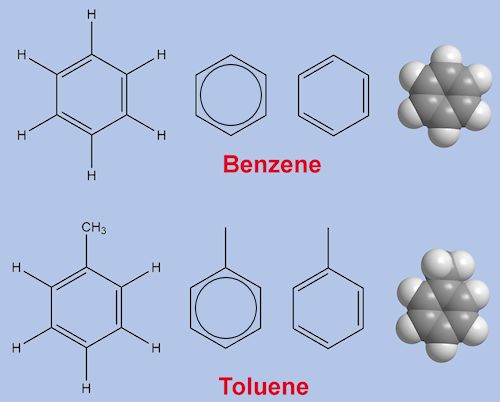
Let’s start with the boiling points. The two hydrocarbons are similar, but toluene (C7H8) is slightly larger, containing more atoms than benzene (C6H6). This leads to stronger van de Waals intermolecular attractive forces, so it takes more energy to separate toluene molecules and vapourise them, hence its higher boiling point.
Benzene molecules are quite flat, so that they stack together well in a solid lattice. The –CH3 group in toluene gets in the way of stacking, toluene molecules do not fit together as well in a lattice, leading to weaker intermolecular forces, and a lower melting point than benzene.
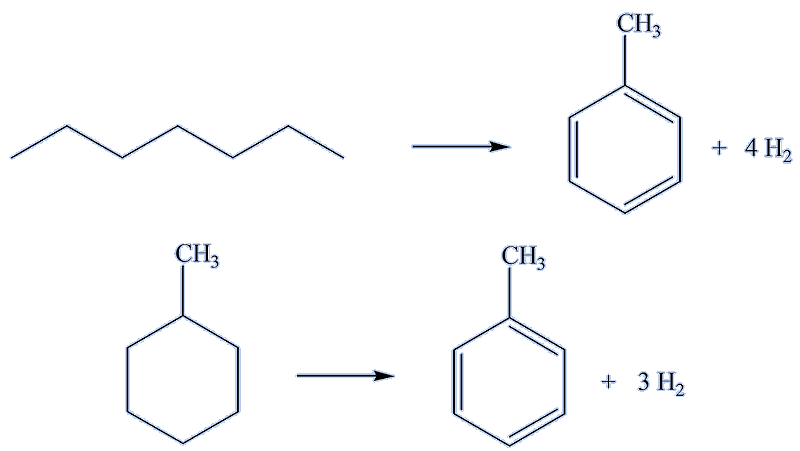
How is it manufactured?To manufacture toluene, the chemical industry uses some of the naphtha fraction from oil refining, which contains a mixture of alkanes with between 6 and 10 or 11 carbon atoms (in some parts of the world, cyclkoalkanes make a substantial component of crude oil.) Industry now uses the process called reforming, typically with platinum- or rhenium-based catalysts - to convert straight-chain alkanes into a mixture of branched alkanes and cycloalkanes, the latter then being dehydrogenated to aromatic hydrocarbons, substantially a mixture of benzene, toluene and xylenes (dimethylbenzenes). Other catalysts convert straight-chain alkanes into arenes. Of course, these mixtures need to be separated by fractional distillation. Hydrogen is a valuable by-product. |
 Oil refinery [Photo: Kendash1987, CC BY-SA 4.0 via Wikimedia Commons] |
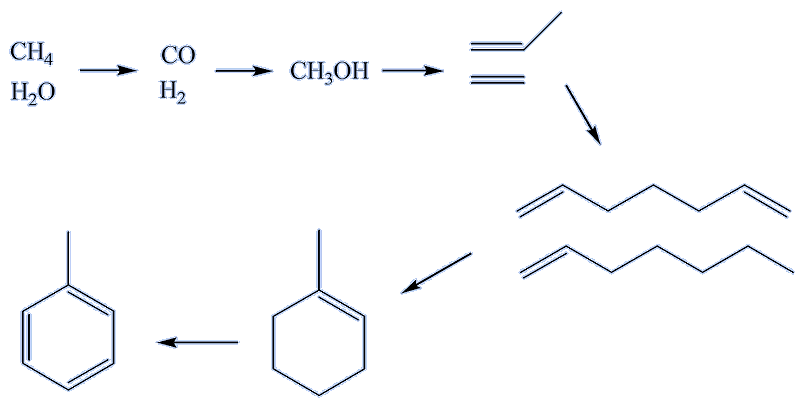
Elsewhere, methanol, made from 'synthesis gas', is converted into aromatics.
Synthesis gas itself comes from routes such as methane reacting with steam, using a suitable catalyst. This can be transformed into methanol, which in turn is converted, using zinc- or gallium-modified ZSM-5 zeolite catalysts, successively into alkenes, cycloalkenes and aromatics, including toluene.
Halogenation (particularly with Cl2) of the methyl side-chain occurs in the presence of ultraviolet light, just as it does in methane. The other significant reaction is oxidation of the side chain. Under mild conditions, the aldehyde benzaldehyde is formed, whilst under forcing conditions (e.g. KMnO4) the product is benzoic acid.
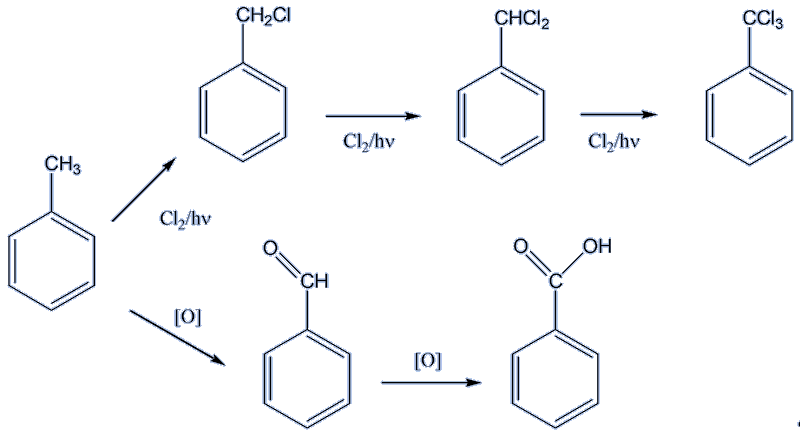
Toluene undergoes similar electrophilic substitution reactions in its ring to benzene. One significant difference is caused by the presence of the methyl group. Alkyl groups such as methyl are electron-donating, so that the benzene ring becomes more electron-rich, and therefore more attractive towards electrophiles, thus reacting faster. Another consideration is that the methyl group directs incoming electrophiles preferentially to the 2- and 4- positions.
You have to look at the structures of the intermediate species formed during the electrophilic substitution reactions. These forms are known as Wheland intermediates. For each substitution – whether giving the 1,2-, 1,3- or 1,4- product – there are three different ‘resonance forms’.
In the case of 1,2- substitution, one ‘resonance form’ – the one on the right in the diagram below (shown in red), has three carbon atoms bound to the carbon bearing the + charge, making it a tertiary carbocation, and so especially stable.
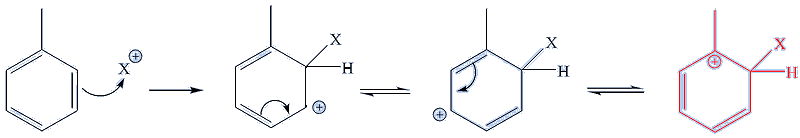
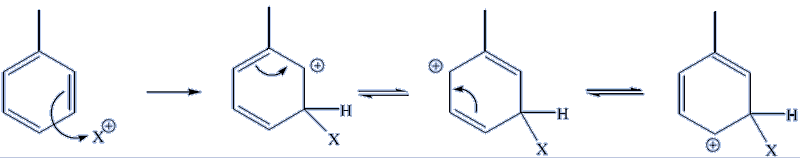
In the case of 1,3- substitution, none of the three resonance forms is a tertiary carbocation, so there is no specially stable species, making 1,3- substitution a less favoured pathway energetically.
For the 1,4- substitution pathway below, again there is one tertiary carbocation among the three resonance forms (the middle one of the three forms, shown in red), again making this route favoured, like the 1,2- pathway.
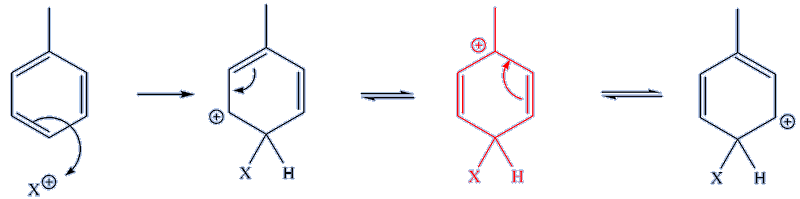
Thus, more of the 1,2- and 1,4- isomers are expected in the final products of the reaction, as is observed in practice.
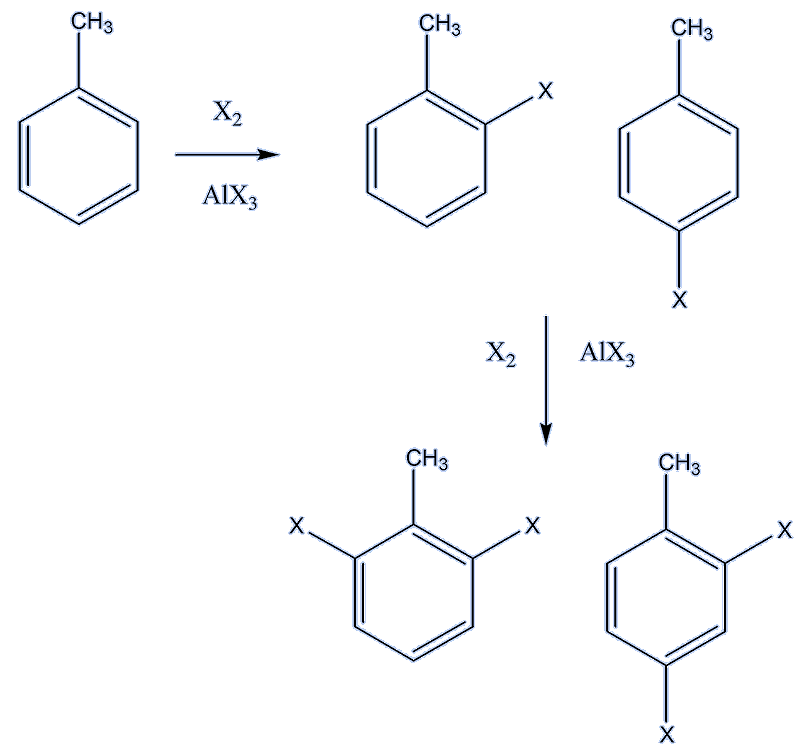
Halogenation with chlorine and bromine requires the presence of a Lewis acid catalyst (e.g. FeCl3, AlX3 (X = Cl, Br), the electrophile being X+. About 60% of the monochlorination product is 2-chlorotoluene and 40% 4- chlorotoluene.
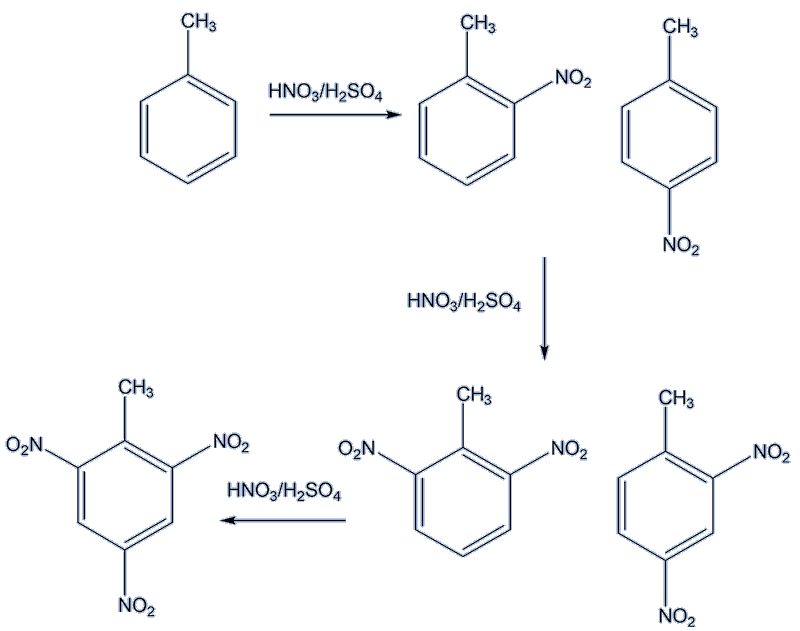
Using a mixture of concentrated nitric and sulfuric acids, nitration occurs faster than it does with benzene. Up to three nitro groups can be inserted; the trisubstituted product 2,4,6-trinitrotoluene being better known as the explosive TNT (MOTM Dec 2014). |
 TNT crystals [Photo: Daniel Grohmann, CC BY-SA 3.0 via Wikimedia Commons] |
The electrophile is NO2+. With mono-nitration, the product is about 60% in the 2-position, just 4% in the 3-position and some 37% in the 4-position.
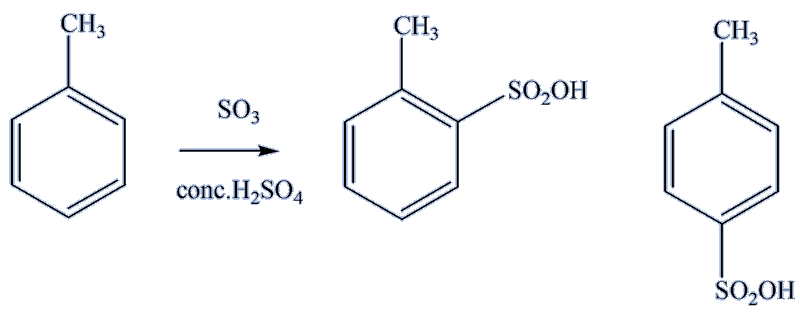
Sulfonation using SO3/conc. H2SO4 produces sulfonic acid, predominantly the 4-sulfonic acid product; the electrophile is SO3 or SO3H+.
What use is toluene?Well, it is widely used as a solvent, not least because it is less toxic than benzene. It is a valuable chemical starting material for the synthesis of other substances. In addition, it is widely used in petrol, as pure toluene has a high octane rating of 114, so is used to improve the octane rating of petrol; indeed, it has been used in the fuel employed in Formula 1 racing. |
 C60 solution in toluene [Photo: Alpha six from Germany, CC BY-SA 2.0 via Wikimedia Commons |
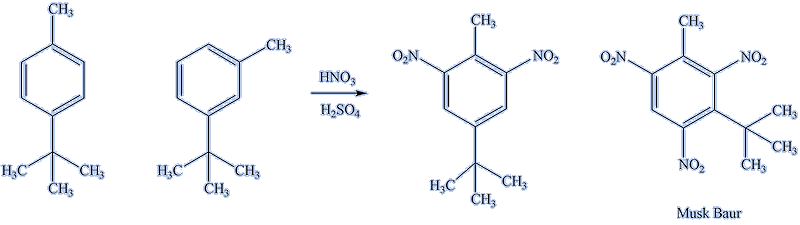
Not all compounds with three nitro groups are explosives; some are significant in perfumery. As long ago as 1758-1759, Andreas Sigismund Marggraf (1709-1782) reacted nitric acid with the rectified oil of yellow amber and obtained a substance with a strong musk smell. It is now believed that the substance he made is a trinitrated meta-cymene (3-isopropyltoluene):
Over a century later, Albert Baur while investigating TNT derivatives as explosives, nitrated isomeric butyltoluenes. He had alkylated toluene with isobutylbromide in a Friedel-Crafts reaction, and only much later realised that the iso-butyl group isomerised to tert-butyl. He then nitrated the resulting mixture of butyltoluenes. The compound now known as “musk Baur”, then “künstlicher Moschus”, crystallised from the mixture. It showed no particular explosive properties, but had a strong musk smell. Baur had the acumen to patent his discovery, which formed the first of a number of nitromusks which were for over half a century to form an essential ingredient of many perfumes, including Chanel Number 5 (MOTM November 2008). |
 [Photo: arz, Public domain, via Wikimedia Commons] |
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.16529535]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.16529535]