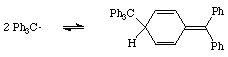
 The NMR shows 25 aromatic protons at 6.8 - 7.4 ppm 4 olefinic protons between 5.8 - 6.4 ppm a single aliphatic proton at 5 ppm |
|
The matter was resolved in 1968, when it was demonstrated conclusively by nmr spectroscopy that the dimer had the quinonoid structure shown below [4]. It appears that the triphenylmethyl radical is too crowded round the central alpha-carbon atom for hexaphenylethane to be formed; instead a bond is formed between the alpha-carbon atom of one radical with the less hindered para-carbon atom of one of the phenyl groups of the second radical.
 The NMR shows 25 aromatic protons at 6.8 - 7.4 ppm 4 olefinic protons between 5.8 - 6.4 ppm a single aliphatic proton at 5 ppm |

|
References
1. J. Piccard, Annalen, 1911, 381, 347; . K. Ziegler and L. Ewald, ibid., 1929, 473, 163.
2. E. Muller and I. Muller-Rodloff, Annalen, 1936, 521, 89; M. F. Roy and C. S. Marvel, J. Am. Chem. Soc., 1937, 59, 2622.
3. C. S. Marvel, J. F. Kaplan and C.M. Himel, J. Amer. Chem. Soc., 1941, 63, 1892.
4. H. Lankamp, W. Th. Nanta and C. MacLean, Tet. Letters, 1968, 249.