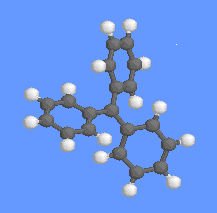
 The suggestion that triphenylmethyl has a "windmill" or propeller" shape was made by Lewis, Lipkin and Magel [1] who measured the UV absorption spectrum. The twisting is a compromise between the ortho-ortho steric repulsion involving aromatic hydrogens on adjacent rings and maximum resonance stabilization which favours a planar structure.
The suggestion that triphenylmethyl has a "windmill" or propeller" shape was made by Lewis, Lipkin and Magel [1] who measured the UV absorption spectrum. The twisting is a compromise between the ortho-ortho steric repulsion involving aromatic hydrogens on adjacent rings and maximum resonance stabilization which favours a planar structure.A gas phase electron diffraction study [2] and X-ray investigations of tris(p-nitrophenyl)methyl [3] and tris(3,5-di-tert-butylphenyl)methyl [4] suggest that the angle of twist is ca. 350.
ESR studies of 13C coupling constants indicate that twisting of the phenyl substituents also occurs in solution [4].
Back to main menu