![]()
![]()
Simon Cotton
Uppingham School, Rutland, UK
![]()
Also available: HTML-only, Chime Enhanced and Jmol versions.
![]()
 You mean the stuff put in the bombs?
You mean the stuff put in the bombs?No, that is usually uranium metal.
No, first you have remove impurities from the uranium compound in the ground (uranium is too reactive to occur as lumps of metal, like gold does). Then when you have pure uranium oxide (often called "yellowcake"), this has to be turned into uranium metal. When you have done that, you need to separate the two sorts of uranium atom; it has been estimated that starting from 50,000 tons of uranium ore you can get less than a ton of the kind of uranium atom needed to make a bomb.
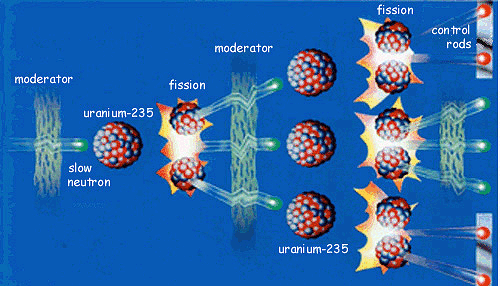
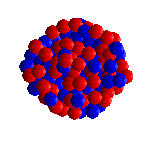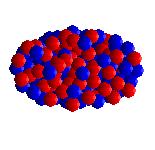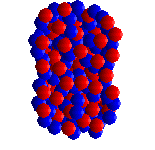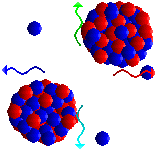
Atom bombs (and nuclear power stations) get their energy from fission of uranium-235 atoms. Neutrons can split uranium-235 atoms up (fission) into two smaller atoms and release more neutrons. So one reaction that can occur when a neutron of the right energy splits a uranium-235 atom is:
1n + 235U ![]() 140Ba + 93Kr + 3 1n
140Ba + 93Kr + 3 1n
These three neutrons can go on to split three more uranium atoms, producing nine more neutrons; this can continue to produce a self-sustaining chain reaction.
Einstein was the first scientist to talk about mass and energy being interconvertible. His famous equation is :
E = m c2
(where E is the energy, m is the mass, and c is the velocity of light).
The total mass of all the particles produced in the fission reaction is a little less than that of a 235U plus a neutron; the difference is released as energy. So each time a uranium-235 is split, a chunk of energy is released. The more atoms split, the more energy is released. If the chain reaction gets out of control, so much energy is produced that a nuclear explosion would occur, and this is how an atom bomb works. To see an animation of this process, click here.
 Does this work with all uranium atoms?
Does this work with all uranium atoms?No. Natural uranium is 99.28% 238U and 0.72% 235U. Only the 235U atoms undergo this kind of fission.
Both uranium atoms have 92 protons; 238U atoms have 146 neutrons and 235U atoms have 3 less.
 So why don't nuclear power stations explode, just like atom bombs?
So why don't nuclear power stations explode, just like atom bombs?Nuclear power stations have to have effective control devices (such as the control rods in the fission diagram earlier) that regulate how many neutrons are flying round the reactor, by absorbing any excess when the fission process is getting too fast.
Heat is removed from the nuclear reactor by a circulating coolant; the hot coolant is used to boil water, making steam. This drives a turbogenerator to make electricity.
 What is all the fuss about nuclear waste?
What is all the fuss about nuclear waste?The atoms formed when uranium atoms are split up are usually very radioactive. The "used" fuel rods from a reactor [discarded when about 25% of the uranium has undergone fission] are kept in a cooling pond for months for the more intensely radioactive atoms to decay and release most of their energy. Then they have to be processed to separate "unused" uranium atoms from the remaining fission products that have to be stored safely in barrels, often in underground bunkers (see photo, right).
In 1972, it was discovered that the uranium content in a mine at Oklo in Gabon (Africa) was very low in 235U. The only acceptable explanation is that some time in the past millions of years ago pockets of ore were formed that contained enough 235U for natural nuclear reactors to be formed (235U made up 3% of natural uranium at that time). Isotopes of fission products of 235U have been found in the ore, in just the exact amounts expected to confirm this theory.
Its main use lies in the separation of the two main isotopes of uranium; you may remember that the most abundant isotope, 238U, does not readily undergo nuclear fission, but the 0.71% of 235U readily undergoes fission with slow neutrons.
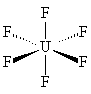 |
UF6 is octahedral, with all 6 fluorines pointing towards the corner of an octahedron. Click on the images to see the 3D structure files. | 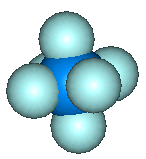 |
If you want a compound of an element in a high oxidation state, fluorine is the most likely element to help you achieve it; partly favoured by the low F-F bond energy, partly because fluorine forms strong bonds with almost any other element. So uranium isn't the only element to form a hexafluoride. Other actinides like Np and Pu do it; several of the platinum metals like Pt, Ru, Os, Rh and Ir form them, so do Tc, Re, Mo and W; and of course there are the famous noble-gas compound XeF6 and the unreactive gas SF6 (important in the electrical industry as an insulating gas and recently the subject of concern for its potential in global warming) as well as SeF6 and TeF6 . But UF6 is the most important, as we shall see.
It is a white crystalline solid at room temperature (its triple point is 64°C (147.3°F) and it sublimes at 56.5°C (133.8°F) at atmospheric pressure). The liquid phase only exists under pressures greater than about 1.5 atmospheres and at temperatures above 64°C (see the phase diagram).
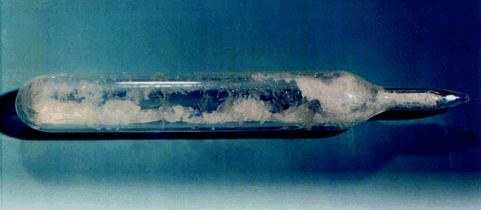
UF6 crystals in a glass vial. (from http://web.ead.anl.gov/uranium/guide/uf6/index.cf)
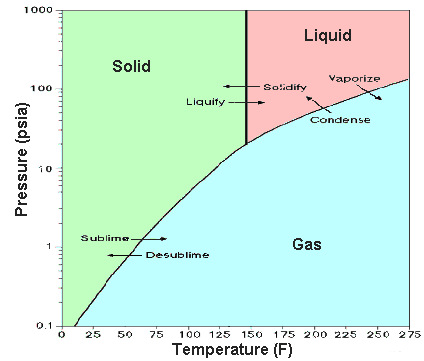
Phase diagram, showing relationship between pressure, temperature, and physical form (from http://web.ead.anl.gov/uranium/guide/prodhand/sld022.cfm).
Like all the other hexafluorides (except XeF6) it has an octahedral structure (U-F = 1.994 Å), with strong covalent bonds within the molecules but only weak Van der Waals' forces between neighbours, so that it has a low boiling point and melting point. Vibrational frequencies are n1 (R) 667 cm-1; n2 (R) 535 cm-1; n3 (IR) 624 cm-1; n4 (IR) 186 cm-1; n5 (R) 201 cm-1; n6 (inactive) 140 cm-1.
It does not react with dry air, oxygen, nitrogen or carbon dioxide. However, when it comes in contact with water, even traces, it undergoes hydrolysis to uranyl fluoride (UO2F2) and the corrosive and toxic gas HF.
UF6 + 2 H2O ![]() UO2F2 + 4 HF
UO2F2 + 4 HF
For this reason, it attacks glass unless absolutely free of either water or HF. It reacts with many elements (e.g. Group I and II metals; B, Al, Ga, In, C, Si-Pb etc) forming their fluorides. Most hydrogen-containing compounds react forming HF, so that hydrocarbons (including arenes) form a mixture of carbon fluorides, carbonaceous material and HF, but there is no reaction with fluorocarbons such as Teflon. It attacks many metals, except Ni (owing to the formation of a surface coating of fluoride) and Al (that bears a surface oxide layer).
There are many ways of making UF6. Although reaction of uranium and fluorine was first noted by Henri Moissan, first isolator of fluorine, c.1900, UF6 was originally reported by Ruff (pioneer of syntheses of many metal fluorides) and Heinzelmann in 1911. They made it by fluorination of uranium, and also by fluorination of UC2; fluorine needs to be heated to react with uranium, unless it is finely divided.
U (s) + 3 F2 (g) ![]() UF6 (g)
UF6 (g)
UC2 (s) + 7 F2 (g) ![]() UF6 (g) + 2 CF4 (g) (at 350°C)
UF6 (g) + 2 CF4 (g) (at 350°C)
An unusual reaction which does not use fluorine (but which has not been employed commercially) is:-
2 UF4 (g) + O2 (g) ![]() UF6 (g) + UO2F2 (g)
UF6 (g) + UO2F2 (g)
The main process used industrially employs fluorination of UF4 at around 500°C. It is a very exothermic process; temperatures can reach 1100°C in reactors with capacities of up to 380 kg per hour.
UF4 (g) + F2 (g) ![]() UF6 (g)
UF6 (g)
Indeed, and its synthesis from the uranium ore is quite a complicated process. The uranium is usually present in the ore as an oxide, approximating to U3O8. This is dissolved in nitric acid forming UO2(NO3)2.6H2O. (In some places, carbonate leaching, affording [UO2(CO3)3]4-, is used). This is purified by extraction using a solvent such as tributylphosphate in kerosene; after evaporation of the solution, the resulting solid is roasted to form UO3. The resulting UO3 is then converted into UF6 in a three-stage process.
It is extremely volatile (it has a vapour pressure of around 120 mmHg at room temperature) so can be handled as a gas for the isotope separation.
Several methods have been used:-
Electromagnetic isotope separation, using UCl4, was the original method used in the Manhattan project in a plant at Oak Ridge Tennessee. Laser isotope separation has been little used, the best known version being the Australian Silex process. Of the other two processes, something over 90% of the world's enriched uranium is obtained this way.
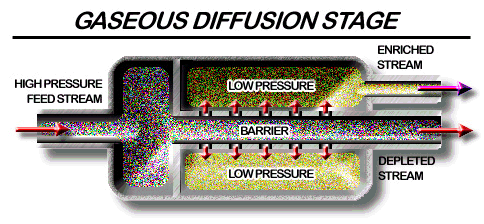
Gas diffusion for isotope enrichment.
From http://www.fas.org/nuke/intro/nuke/uranium.htm
In gaseous diffusion, UF6 gas is forced to diffuse under pressure through porous membranes. The lighter 235UF6 molecules diffuse slightly faster than the 238UF6 molecules. As the gas moves, the two isotopes are separated, increasing (enriching) the U-235 concentration, and decreasing (depleting) the concentration of U-238. However, over 1000 stages are required to produce a UF6 product with even 3-4% enrichment! As the most volatile uranium compound, UF6 was an obvious choice for use, but because of its high reactivity and problems associated with its handling, many alternatives were investigated, such as alkoxides, whilst unsuccessful attempts were made by Gilman to obtain simple alkyls. Ultimately the technology was improved to enable full advantage to be taken of its volatility.
As an a-emitter, uranium is not especially hazardous unless ingested by the body. However, the ready reaction of UF6 with water makes it a potentially hazardous substance, owing to the toxicity of the HF produced. In a 1944 accident when a cylinder containing UF6 was being heated by steam, a weld ruptured, releasing some 400 lb of UF6; the reaction of UF6 with steam released sufficient HF to cause two fatalities. Another person was killed by inhaled HF in 1986 following rupture of a heated cylinder; some 31 other people were exposed to the fumes but no one else appears to have suffered long-term ill effects.
![]()
![]()
![]() Back to Molecule of the Month page.
Back to Molecule of the Month page.