 |
 |
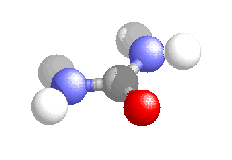
It was synthesised in 1828 by Friedrich Wohler and was the first organic compound to be synthesised from inorganic starting materials. It was found when Wohler attempted to synthesis ammonium cyanate, to continue a study of cyanates which he had be carrying out for several years. On treating silver cyanate with ammonium chloride solution he obtained a white crystalline material which proved identical to urea obtained from urine.
This discovery prompted Wohler to write triumphantly to Berzelius:-
"I must tell you that I can make urea without the use of kidneys, either man or dog. Ammonium cyanate is urea."
This organic systhesis dealt a severe blow to a widespread belief called "vitalism" which maintained that organic chemicals could be modified by chemistry but could only be produced through the agency of a vital force present in living plants and animals.
In 1870 urea was produced by heating ammonium carbamate in a sealed vessel. This provided the basis of the current industrial process for its production.

This was discovered by Adolf Bayer in 1864. But the barbiturates were not
exploited as hypnotics until the early 1900's.
Urea is also used in the production of various acylureas and urethanes for
use as sedatives and hypnotics.
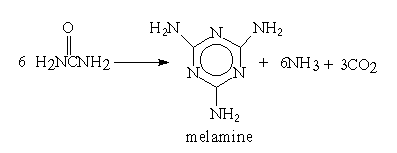
Almost 100 years elapsed between Liebig's discovery in 1834 and a commercial process being developed.
Melamine is primarily used in the production of melamine-formaldehyde resins which have much greater hardness and stain resistance than urea-formaldehyde resins.
Both melamine-formaldehyde and urea-formaldehyde have very varied uses including adhesives, laminates, moulding compounds, coatings and textile finishes.
Its action of nitrogen release is due to the conditions favouring the reagent side of the equilibriums which produce urea.
As the helices are interconnected all helices in a crystal must have the same 'handedness'. This is determined when the crystal is nucleated and can thus be forced by seeding. This property has been used to separate racemic mixtures.
For historical information:-
PARTINGTON J.R., (1962), A History of Chemistry, MacMillan, London, Vol. 3.
PARTINGTON J.R., (1964), A History of Chemistry, MacMillan, London, Vol. 4.
These are good sources with the information collated via the scientists.
For information on channel compounds of urea (not for the faint hearted):-
PARSONAGE N.G. and STAVELEY L.A.K, (1978), Disorder in Crystals, Clarendon Press,
Oxford, pp.756-779.
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5469661]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5469661]