|
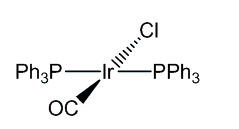
Vaska's Compoundtrans-chlorocarbonylbis(triphenylphosphine)iridium(I)
|  |
|
Vaska's Compoundtrans-chlorocarbonylbis(triphenylphosphine)iridium(I)
|  |
 How come this compound is named after someone?
How come this compound is named after someone?Lauri Vaska, a professor of Chemistry at Mellon University in Pittsburgh in the 1960s, wasn't the first person to make it, but he was the person to correctly formulate it, and then to discover that it had some interesting and, indeed, important reactions.
It reacts reversibly with O2 and with H2, and as such was one of the first compounds discovered that mimics the action of haemoglobin. However, the product of the reaction with dioxygen contains an O2 molecule bound side-on, unlike the end-on O2 molecule when haemoglobin binds dioxygen.
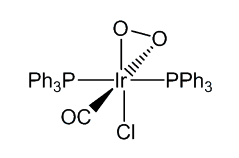
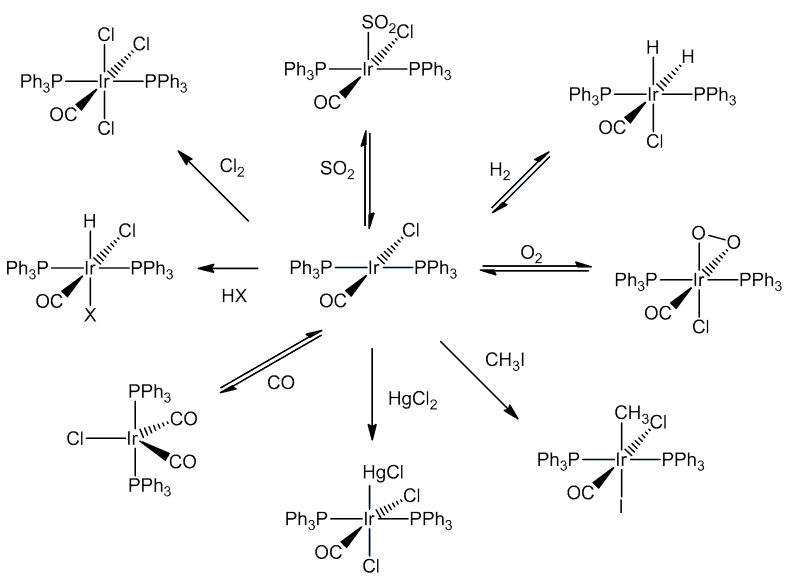
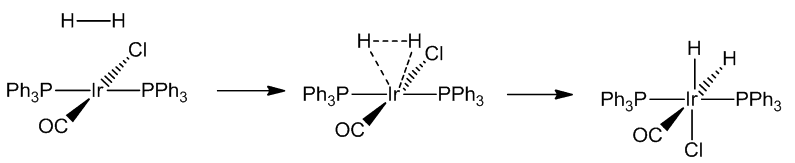
[IrCl(CO)(PPh3)2] similarly reacts with a wide variety of molecules including Cl2, HCl and CO, as well as SO2 and HgCl2. These reactions are generally known as oxidative addition reactions.
Oxidative addition reactions are common in transition-metal chemistry, especially compounds with 16-electron configurations - notably square-planar complexes of Rh(I) and Ir(I). In these reactions, both the coordination number and oxidation state of the metal increase by two, as does the electron count, so that they now obey the 18-electron rule.
Complexes of transition metals in low oxidation states with strong π-acceptor ligands, notably CO, have nine orbitals in their "valence shell", which can hold 18 electrons.
The changes are accompanied by a shift in the position of the CO stretching frequency in the IR spectrum.
| Molecule added | Formula of product | νCO |
|---|---|---|
| - | IrCl(CO)(PPh3)2 | 1967 |
| O2 | IrCl(CO)(O2)(PPh3)2 | 2015 |
| SO2 | IrCl(CO)(SO2)(PPh3)2 | 2021 |
| HCl | IrHCl2(CO)(PPh3)2 | 2046 |
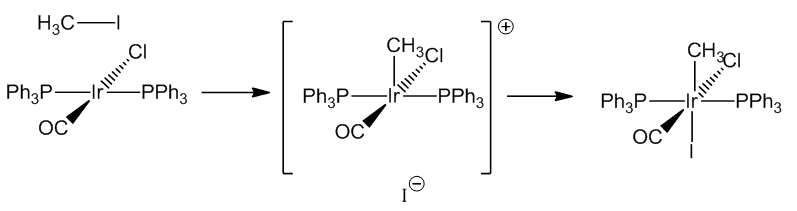
| CH3I | IrCl(CH3)I(CO)(PPh3)2 | 2047 |
| Cl2 | IrCl3(CO)(PPh3)2 | 2075 |
| D2 | IrClD2(CO)(PPh3)2 | 2034 |
| CO | IrCl(CO)2((PPh3)2 | 1934, 1988 |
Bonding in carbonyl compounds involves both CO-to-metal σ-donation and metal-to-ligand π-backbonding: in the latter. Electron density goes from filled metal d orbitals into empty π antibonding ligand orbitals.
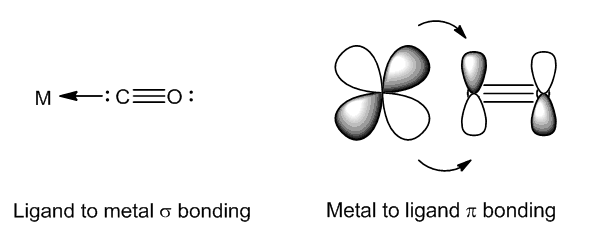
As the oxidation state of iridium increases, there are fewer d electrons available for backbonding, thus the CO bond becomes stronger and the value of νCO increases. So when one molecule of CO adds to form [IrCl(CO)2(PPh3)2], the compound is still Ir(I) and the average CO stretching frequency is hardly changed, but when one molecule of Cl2 adds, forming the Ir(III) compound [IrCl3(CO)(PPh3)2], there is a shift in νCO of over 100 cm-1.
Many studies have been made of the mechanisms. In some cases the incoming molecule is attached without breaking up the integrity of the molecule concerned (e.g. O2, SO2):

In some cases, the concerted addition is followed by breaking up of the original molecule (e.g. H2, Cl2):
The addition of methyl iodide proceeds by a SN2 reaction with the slow step involving a five-coordinate cationic species.
 What does IrCl(CO)(PPh3)2 look like?
What does IrCl(CO)(PPh3)2 look like?IrCl(CO)(PPh3)2 is a bright yellow crystalline solid. It is soluble in hydrocarbons like benzene or toluene, as well as chloroform, but insoluble in ethanol.
An obvious way is by a substitution reaction of IrCl(CO)3 with PPh3:
[IrCl(CO)3] + 2 PPh3 ![]() [IrCl(CO)(PPh3)2] + 2 CO
[IrCl(CO)(PPh3)2] + 2 CO
More commonly, though, it is made by heating an iridium compound like IrCl3 or [IrCl6]2- with PPh3 in a solvent that can act as a source of CO, like DMF or 2-methoxyethanol.
![]()
![]()