![]()
Vitamin K
The vitamin required for blood klotting
![]()
![]()
Molecule of the Month May 2016
Also available: HTML version.
![]()

Vitamin KThe vitamin required for blood klotting
Molecule of the Month May 2016
|
 |
You’ve spelled ‘clotting’ incorrectly!Actually it was deliberate. Unlike the other vitamins, A, B, C, etc., which were named alphabetically in the order in which they were discovered, vitamin K was called ‘K’ for a reason. Go on...In 1929, Danish scientist Henrik Dam was studying the role of cholesterol in diet. He did this by feeding chickens a cholesterol-depleted diet, and seeing what this did to the chickens. After a few weeks the chickens started bleeding profusely. However, it wasn’t the missing cholesterol that was causing the problem. In the chemical process to remove the cholesterol from the feedstock, another compound had also been inadvertently removed. When this compound was added back to the feedstock, the chickens became normal. Dam realised that the mystery compound was necessary for blood clotting, and so called it the ‘coagulation vitamin’. The new discovery was then reported in a German Journal, where it was called Koagulationsvitamin, and thereafter renamed in English as Vitamin K. Dam worked with American scientist Edward Doisy to work out the biological effects of Vitamin K as well as its structure. They both shared a Nobel Prize in Medicine for this work. |
|
|
Actually, ‘vitamin K’ is the name given to a group of similar molecules that all act in the same way. They are essential vitamins that the body needs in order to make the proteins for blood clotting.
There are 5 types of vitamin K, denoted K1-K5, although only K1 and K2 are naturally occurring; the other three are man-made and are used sometimes as dietary supplements.
|
The K vitamins are all based on derivatives of 2-methyl-1,4-naphthoquinone. The most important source of these is K1, which has a long sidechain (called a phytyl group) attached to the quinone, giving it the chemical name phylloquinone. It’s found in high concentrations in green leafy plants, such as curly kale, spinach, broccoli, and cabbage.
|
|
|
Well, obviously not for clotting blood! Plants actually use vitamin K1 for another role, as an electron acceptor during photosynthesis. In this process, sunlight is captured by chlorophyll molecules, which causes an electron to be excited to a higher energy level. As the electron relaxes back down to its normal level, the excess energy is transferred to nearby molecules (one of which is vitamin K1) allowing them to be either oxidised (lose electrons) or reduced (gain electrons). The electrons are passed along a chain of molecules in a sequence of oxidation-reduction steps, until they are eventually used to drive the reaction turning CO2 and water into sugars and O2.
Not directly. After we eat foods containing vitamin K1, around 75% of the K1 will be converted in our bodies to vitamin K2, which is the real workhorse when it comes to blood clotting. In fact, there are many different sub-types of vitamin K2, depending on the length of the sidechain. The first step in the conversion is to replace the phytyl chain with one containing an unsaturated geranylgeranyl group (4 isoprene units in a line). This new compound is called menaquinone, or MK-4. The ‘M’ stands for menaquinone, the ‘K’ for vitamin K, and the ‘4’ for the four isoprene units (CH2=C(CH3)CH=CH2) in the sidechain.
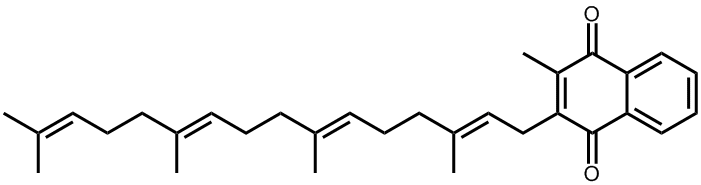
MK-4, the most common type of Vitamin K2 found in animal tissues, is synthesised in the arterial walls, pancreas, and testes.
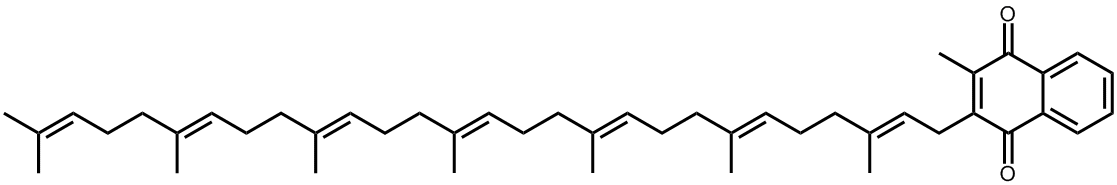
MK-7, with 7 isoprene units, can only be synthesised by bacteria.
Other types of Vitamin K2 exist with different sidechain lengths, such as MK-7, MK-9, etc.; these are not synthesised by animals, but by bacteria in our guts. They undoubtedly assist MK-4 with the bloodclotting process, but by how much, and what their exact role is, is still unclear.
The key chemistry is that the quinone group can be easily reduced and reoxidised, via an epoxide intermediate, using various enzymes. The actual biochemical pathway, called the Vitamin K cycle, is complex, but the net effect is that a COOH group can be added to a nearby protein (such as prothrombin).
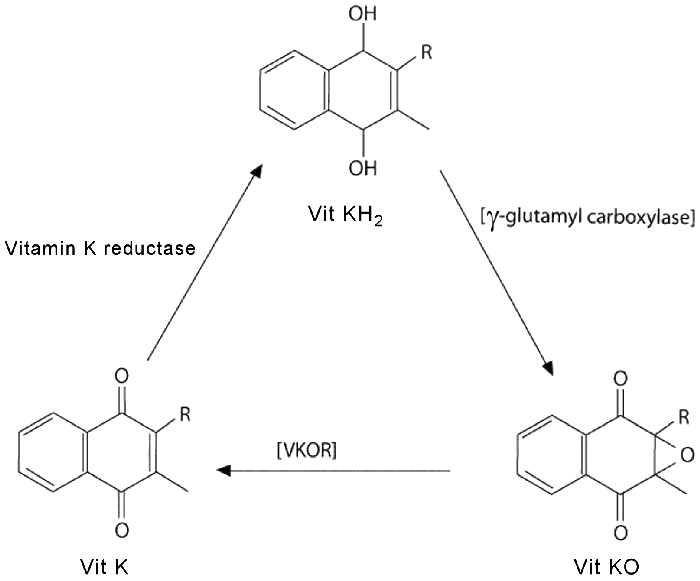
The Vitamin K cycle.
Vitamin K is reduced in the liver by enzyme Vitamin K reductase to its hydroquinone form, vitamin KH2. This then catalyses the carboxylation of the nearby protein (via the enzyme gamma-glutamyl carboxylase) and is converted to its epoxide (vitamin KO). This is then converted back to vitamin K by the enzyme Vitamin KO reductase (VKOR). As you can see, the vitamin K acts as a catalyst, which means that only a tiny concentration is needed to produce a large effect.
The proteins involved in blood coagulation, such as prothrombin, have at their amino terminus, typically 9 to 12 glutamic acid sidechains. The vitamin K cycle adds a second COOH group to the end of the chain, to make a so-called gamma-carboxy-glutamic acid.
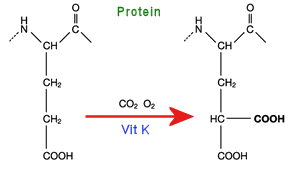
The two adjacent COOH groups now make the protein extra ‘sticky’, especially to calcium ions, which can now chelate (bond) to them directly. The trapped calcium ion is now able to form an ionic bond to the phosphate head-groups of phospholipid membrane surfaces.
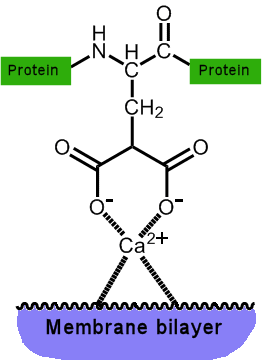
Once the protein (prothrombin) is stuck to the lipid wall, another enzyme can convert it to thrombin, whose key role is to cut up fibrinogen, an inactive circulating plasma protein, into soluble fibrin monomers. These reactive monomers then spontaneously aggregate to form a spaghetti-like network, clotting the blood. The other useful effect that results from the binding of Ca2+ ions is that these ions are removed from the bloodstream (where they might eventually clump together and form calcified blockages of arteries), and can be donated to proteins that can do something useful with them, such as make bones. Indeed, one of the symptoms of vitamin K deficiency is weak bones, while one of the treatments for osteoporosis is to take vitamin K supplements. |
|
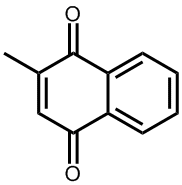
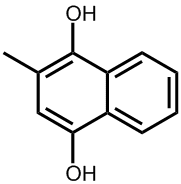
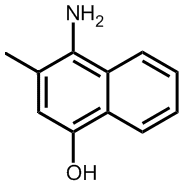
Ah, these are the man-made analogues called vitamin K3, K3 and K3. Because these are not found in nature, strictly speaking they are not true vitamins, but due to their similarity in structure and effect to vitamins K1 and K2 they have co-opted the name. They are synthesised mainly for use as nutritional supplements for use by people with illnesses or ailments that cause vitamin K deficiency.
 |
 |
 |
Vitamin K3 (menadione) |
Vitamin K4 (menadiol) |
Vitamin K5 |
A healthy adult with a normal diet can normally get all the vitamin K needed from eating a few green vegetables regularly. But newborn babies are relatively deficient in vitamin K because they have low vitamin K stores at birth. This is because the levels of vitamin K in breast-milk are low, and what little there is does not pass easily through the placenta. Also, a baby’s gut bacteria have not yet been developed so they cannot synthesise their own vitamin K2. This puts newborns at risk of ‘Haemorrhagic disease of the newborn’ (excessive or uncontrolled bleeding), so to prevent this many newborns are given a vitamin K supplement at birth.
If a person has damage to the liver or gut where vitamin K2 is synthesised, then they may have a deficiency also, and become susceptible to uncontrolled bleeding. Patients that take warfarin are also at risk.
It’s a drug that’s used to prevent blood clotting in patients where excessive clotting is dangerous, especially in areas of slowly running blood such as in veins and the pooled blood behind artificial and natural heart valves. Commonly, warfarin is used to treat ailments such as atrial fibrillation, deep-vein thrombosis, and pulmonary embolism.
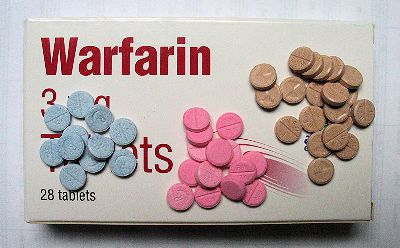 |
|
Test |
Warfarin tablets 3 mg (blue), |
Above and right: The structure of warfarin. |
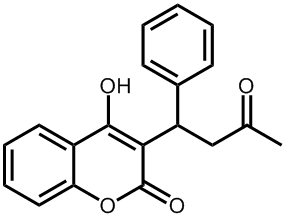 |
It works by inhibiting vitamin K epoxide reductase (VKOR), the enzyme that converts vitamin KO back to vitamin K in the vitamin K cycle, above. This means the vitamin K is not regenerated, and as it is used up so its concentration falls, and the blood-clotting mechanism slows. People who are on warfarin treatment are usually advised to be very careful about how many green leafy vegetables they eat, as their high vitamin K content can negate the effect of the warfarin – or make it difficult for the body to regulate how much vitamin K is present at any one time.
Yes, because it prevents rats’ blood from clotting too. This means that after eating warfarin-laced food bait, any slight scratch, scrape or injury that the rat might experience causes it to bleed to death. Nowadays, though, the use of warfarin as a rat poison is declining because many rat populations have developed resistance to it. It’s being replaced by more potent and longer acting poisons such as coumatetralyl and brodifacoum [see the MOTM page on warfarin].
![]()
![]()