Experiment 4 The Preparation and Properties of Organo-cobaloxime Complexes
Version of October 2002 - These were the instructions whilst the experiment ran in the Bristol 2nd Year Inorganic teaching lab until May 2002, the experiment was then withdrawn.
Dr John Maher, School of Chemistry, Bristol University
E-mail: Tel: +44 (0) 117 (928) 7653
Introduction
Just as the tetrahedron is an icon of organic chemistry, so the octahedron is an icon of inorganic chemistry, and because Co(III) complexes are easy to study and prepare, a very large proportion of our knowledge of inorganic isomerism, reaction mechanisms, and the general properties of octahedral complexes is based upon studies of Co(III) chemistry.1 In Co(III) complexes the cobalt atom shows an affinity for nitrogen donor ligands, moreover these are mostly substitution inert i.e. non-labile. The slow substitution rates made the complexes amenable to study before the development of modern instrumental methods. In Co(I) and Co(II) complexes the ligands are labile (rapidly substituted), thus the normal mode of preparation for Co(III) complexes is to form a Co(II) or Co(I) version of the required complex, then to oxidise it to its Co(III) equivalent.
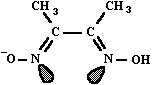 The dimethylglyoxime monoanion (dmgH- ), a strong chelate ligand, forms Co(III) complexes [Co(dmgH)2AX], A = NH3, py, PPh3; X = Cl-, Br-, I-, NO2-, SCN-, N3-, R-. Thus A is a neutral N, P, O, or S donor Lewis base ligand, and X is a mono-anion; X can also be a carbanion, R-, giving organometallic complexes such as [CH3Co(dmgH)2A] . Referred to as a cobaloxime complex, the latter compound can be called 'methylcobaloxime'. Cobaloxime complexes in oxidation states Co(I) to Co(IV) are known.
The dimethylglyoxime monoanion (dmgH- ), a strong chelate ligand, forms Co(III) complexes [Co(dmgH)2AX], A = NH3, py, PPh3; X = Cl-, Br-, I-, NO2-, SCN-, N3-, R-. Thus A is a neutral N, P, O, or S donor Lewis base ligand, and X is a mono-anion; X can also be a carbanion, R-, giving organometallic complexes such as [CH3Co(dmgH)2A] . Referred to as a cobaloxime complex, the latter compound can be called 'methylcobaloxime'. Cobaloxime complexes in oxidation states Co(I) to Co(IV) are known.
A fascinating development was the discovery in 1962, from an X-ray crystal structure determination by Dorothy Hodgkin, that the Vitamin B12 coenzyme contained a Co-C s-bond.2 Chemical studies have since shown that this bond is non-labile yet at the same time a relatively weak bond. The discovery of the alkylcobaloximes occurred at about the same time, so that they were adopted at an early stage as cobalamin (Vitamin B12) models.
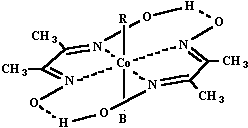 The chelate ligands around the cobalt(III) can vary, as can R and B {R = alkyl group, B = Lewis base} so that large numbers of organocobalt complexes have now been prepared and studied, both as models for B12 chemistry, and in their own right as interesting compounds. In this experiment you will prepare, and then study some properties of one of the alkylcobaloximes. 3
The chelate ligands around the cobalt(III) can vary, as can R and B {R = alkyl group, B = Lewis base} so that large numbers of organocobalt complexes have now been prepared and studied, both as models for B12 chemistry, and in their own right as interesting compounds. In this experiment you will prepare, and then study some properties of one of the alkylcobaloximes. 3
Preparative Section. (Must be carried out by each student individually)
Whilst carrying out this preparation record in your laboratory notebook your observations about the progress of the preparation at its various stages :
- the colour changes that take place in the reaction mixture.
- any general observations on the course of the reactions
- any difficulties/problems encountered with the preparation
- your % yield
Quantities needed for the preparation
| Quantity | Moles | Compound |
|---|---|---|
| 0.860 g | (0.0075 mole) | Dimethylglyoxime. |
| 0,890 g | (0.00375 mole) | Cobalt(II) chloride hexahydrate. |
| 0.30g | - | Sodium Hydroxide (A). |
| 0.15g |
- |
Sodium Hydroxide (B) |
| 0.29g (0.3ml) | (0.00375 mole) | Pyridine |
| 0.020g | (0.000604 mole) | Sodium Borohydride |
| Calculate this | (0.01mole) | Haloalkane |
| 20 ml | - | Methanol. |
Apparatus
A 50ml 2-neck flask is fitted with a pressure-equalising funnel, N2 bubbler and small magnetic stirrer piece. The 2-neck flask should be clamped by its neck, the clamp should not be attached to the dropping funnel. The flask will eventually be placed in a small plastic cooling bath over the centre of the magnetic stirrer table. The top of the funnel is connected to the nitrogen bubbler. Apply a very small amount of Vaseline to the apparatus joints. After mixing the Co(II), the ligand and the methanol, the reactions must be conducted under nitrogen, and for formation of the Co(I) cobaloxime, at a temperature of about -10°C.
Method
The quantities used are small so that you should be careful when measuring, in particular the relative amounts of the chemicals are important – small variations seem to lead to large variations in the product yield. Except for the sodium borohydride (weighed on a 4-figure balance) the other chemicals can be weighed with a top-pan 3 figure balance. Use plastic weighing boats for solids (not paper), various small plastic capped vials , measuring cylinders and Pasteur pipette droppers are provided for handling the liquids.
Weigh the Co(II) salt, transfer it into the 2-neck flask using the powder funnel. Remove the powder funnel, and add half of the methanol, stir until the Co(II) salt is dissolved. Weigh the dimethylglyoxime, add this to the flask contents, followed by the remaining methanol (to wash the funnel).
Close the flask with the pressure-equalising dropping funnel and stopper, attach the nitrogen bubbler, keep stirring and pass nitrogen gently through the suspension for about 10 minutes. From now on, the remaining reagent solutions must be added to the dropping funnel by removing the nitrogen tube briefly and using a Pasteur pipette to put the reagent at the bottom of the dropping funnel .
Sodium hydroxide pellets are difficult to measure in small amounts, so that it is easier to work from a stock solution in water (a 15% w/v NaOH solution is provided), you will need 2 ml and 1ml quantities of this solution. Measure the pyridine carefully into a small vial, and dilute with 2-3 ml of methanol.
Put the 2 ml NaOH (Solution A) together with the pyridine solution into the dropping funnel, making sure that the nitrogen is kept running, then slowly, drop-by-drop, add the mixture to the cobalt and dimethylglyoxime, stirring all the time.
When the addition is finished you should have a deep brown-yellow air-sensitive solution, possibly with a slight precipitate. All of the Co(II) and most of the dimethylglyoxime should be dissolved up, there should be no coagulated purple or white pieces at the bottom of the flask.
Prepare a -10°C cold bath by mixing ice (200g) with about 50 ml of ethanol. Leave the flask to stir for about 10 minutes in the cooling mixture. Weigh the sodium borohydride very carefully into a small glass vial, add to it 1 ml of the NaOH (Solution B ), transfer this to the dropping funnel. Slow the rate of nitrogen bubbling slightly. Add the NaOH/NaBH4 solution to the Co(II) mixture. Stir for about a minute. Weigh the haloalkane into a vial, transfer this to the dropping funnel, wash the vial with a couple of mls of methanol and add this also. Add the haloalkane/methanol slowly over a minute or so from the dropping funnel to the (now) Co(I) solution. Stir for about 10 minutes. Remove the low temperature bath and bring the reaction flask back up to room temperature. Stop the nitrogen.
Extraction of Your Product
Warm your product in the 2-neck flask very gently on a steam bath until the methanol is just off the boil. CAUTION METHANOL VAPOUR IS POISONOUS – DO NOT LEAVE BOILING METHANOL FLASKS ON THE STEAM BATH. In any case boiling your product will decompose it! Place a fluted filter paper in the larger plastic powder funnel. Put a couple of mls of methanol into a single neck 100 ml conical flask. Put the funnel with the fluted paper in the neck of this flask and place the flask and funnel on the steam bath, when the methanol at the bottom of the flask is boiling, and the flask and filter system are thoroughly hot, filter your (hot) product solution through the fluted paper. Try to stop the filtrate boiling. With this technique the filtration is conducted under methanol vapour, nevertheless filtration may be rather slow, so be patient! If there is some solid left, then this may dissolve up in a little more hot ethanol, if it is insoluble, then it is an unwanted cobaloxime by-product. In view of the small quantities be careful with your washings and your product, it is easy to get it 'spread out' at this stage, and also easy to knock the flasks over on the steam bath.
Transfer the filtrate to a round bottom flask and evaporate the filtrate with a rotary evaporator, keeping the flask warm with hot water on a steam bath. CAUTION :ALWAYS WEAR SAFETY SPECTACLES WHEN WORKING WITH OR NEAR THE ROTARY EVAPORATOR. Be sure that the flask that you use is not more than one third full - this will help to prevent bumping. Divide the product solution into two parts if necessary. You need to evaporate the methanol/water until a thick muddy mass is obtained - not to dryness.
Precipitate/crystallise the product.
Add 50ml of ice cold water containing 1% by volume of pyridine to the round bottom flask. Put a stopper on it and shake the flask vigorously to dislodge any solid from the flask walls. Allow the crystals to 'improve' for 15-20 minutes in the ice bath. Filter the product through a glass sinter. Dry your sample carefully, drying is crucial to the stability of the product. The sample is best dried by gently drawing air through the crystals on the sinter. Be patient and do not try to stir the contents of the sinter with your spatula whilst it is wet, this will break up the precipitate, slow down the drying process, and you may even get glass from the sinter in your product!
Weigh your product. Calculate the yield. Hand in your sample in a small vial. Your sample bottle must be labelled with : Your Name ; Date; Amount of product; Product Name, e.g. Samantha Plunkett, Dec 2001, 0.95g, pentyl-cobaloxime.
AFTER YOU FINISH, PLEASE CLEAN AND DRY ALL OF YOUR APPARATUS FOR THE NEXT PERSON DOING THIS EXPERIMENT.
Tests and Reactions.
Carry out the following tests, make careful observations. Use the various spectra provided for 4) and 5).
Each student should do test 1), Tests 2) and 3) may be done jointly.
- Reaction of Co(II) with hydroxide. Make up a dilute solution of cobalt(II) chloride in water (approx. 10mg/ml). Add drops of 2M sodium hydroxide from a Pasteur pipette. Shake the solution. Watch what happens over a few minutes. Record your observations.
- Solvent effects on Co(II) solutions. Make up 2 mls of a solution of cobalt(II) chloride in ethanol in a test tube. Now cool the test tube with some liquid nitrogen. You don't need to freeze the tube! Warm the tube to room temperature again. Record your observations.
- Photolysis of methylcobaloxime. Make a saturated solution of the methylcobaloxime complex (available from the hatch), in about 3 ml of water containing a couple of drops of concentrated hydrochloric acid, filter this into a B14 stoppered test tube. Bubble N2 for a few minutes to expel the air, seal the tube quickly with a greased B14 stopper. Support the tube on a clamp stand, leave this in the window to expose the solution to sunlight. When there is very bright sunshine the reaction can be over in minutes, normally the tube will need to be left an hour or even much longer depending on the sunlight level. The effects of photolysis are best observed if the tube is not moved at all. Record your observations of any changes to the solution.
- Photolysis - Spin Trapping , an Electron Magnetic Resonance (EMR) example. Spin trapping is a technique in which a reactive free radical reacts with a double bond of a diamagnetic compound, the spin trap, to form a less reactive and stable free radical, the spin adduct. The technique is used when the primary free radical cannot be detected directly because it is very reactive, and so has a lifetime of microseconds or less. By trapping the radical, the lifetime can be increased to minutes or longer. The EMR spectrum was obtained by spin trapping an aqueous solution of the methylcobaloxime complex, exposed in strong sunlight together with the spin trap PBN, N-tert-Butyl-a-phenylnitrone. The trapping reaction is:
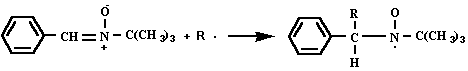
- UV/visible spectra measurements of cobaloxime solutions. Visible spectra, between 300nm and 600nm, of solutions of the methylcobaloxime have been measured:
- Methylcobaloxime complex, aqueous solution only - nothing added
- Methylcobaloxime complex, aqueous solution + concentrated hydrochloric acid
- Methylcobaloxime complex, aqueous solution + excess of pyridine
- Methylcobaloxime complex, in 1,2-dichloroethane solution
Look at the spectra carefully and answer the questions given later.
Write-up and Questions
You must write your report independently, even though some of the work has been done in collaboration with others. Please keep your answers brief and to the point.
- you are not required to re-write the experiment notes!
- you should keep the instruction sheets with your write-up as a reference.
- you must include your observations from the notes that you took during the preparation, and the observations that you made during the tests.
- you must quote your preparation yield (% efficiency to within 1%)
- read the additional experiment notes in the library TP659 to help understand the background.
The Preparation
- .Write a short description of your observations during the preparation. In these give the reactions which are occurring during your preparation, indicating the oxidation state of the cobalt for the different reaction stages. Try to correlate this with your observations about the colour of the solution at the various stages. You can assume the following stages to the chemistry:
- Dissolving the Co(II) chloride in the methanol and adding the ligand
- Under N2, adding the NaOH and the pyridine.
- Cooling the solution and adding more NaOH and NaBH4
- Adding the alkyl halide
Note that the Co(II) dimethylglyoxinate complex, probably [Co(dmgH)2(MeOH)(py)], does not form until step b), it is not formed until the ligand is converted to its anion with NaOH.
- Suggest a mechanism for the formation of the alkylcobaloxime assuming that the cobalt is in the Co(I) state. What is the alternative mechanism if a Co(II) dimethylglyoxinate complex is involved? Hint: Co(I) is a powerful nucleophile and low spin Co(II) behaves like a free radical. The mechanisms also control the reaction yield.
- Describe and explain what happened when you added NaOH solution to the cobalt(II) solution.
- Describe and explain what happened when you dissolved the cobalt(II) complex in ethanol, and then cooled and warmed it again.
- When the photolysis is carried out with a strong solution and under nitrogen, you should observe the formation of a light pink solution and a white precipitate; gas bubbles may also be seen to form. However the rate at which all of this occurs is very dependent on the sunlight level! On a cloudy day the photolysis is very slow. Also, if the photolysis is conducted in air the solution colour becomes light yellow, the precipitate still forms, but no radicals can be trapped from the solution, neither are gas bubbles formed.
- What evidence do you have for the formation of free radicals by the photolysis?
- Suggest inorganic and organic products for the photo-decomposition. Assume that the light photon cleaves the Co-C bond homolytically. Note that alkyl radicals do not react readily with surrounding water molecules.
- Why does the spin-trap not work in the presence of atmospheric oxygen? (No spin adduct is detected by EMR)
- The photo-decomposition only occurs in strong sunlight, a dull cloudy day inhibits photo-decomposition, photolysis is a fine weather experiment! Moreover the reaction takes place behind window glass. What do these facts and the UV/visible spectra tell you about the range of wavelengths of light inducing photo-decomposition, i.e.give the wavelength range over which you think the complex is sensitive (in nanometers, nm). Calculate the energy involved with the upper and lower limits for the wavelength range (in kJ.mol-1) Hint: observe the wavelengths over which the complex absorbs light, and the region over which window glass transmits light.
- Very briefly, explain the observed splitting pattern in the EMR spectrum, estimating the electron-nucleus hyperfine couplings AN and AH (like J couplings in NMR spectra) between the nitrogen, 14N has I=1, and the hydrogen, 1H has I=½, on the adjacent carbon. If hyperfine couplings were observed from the methyl and/or the tertiary butyl group hydrogens, to the electron, describe the EMR splitting pattern that you would you expect to see? Hint: remember NMR and the Pascal triangle.
- Suggest what is happening to the solutions when you add more pyridine on the one hand, or acid on the other. Remember that you add extra pyridine at the end of the preparation; this observation helps to explain why extra pyridine is added at this stage. Hint: compare the spectra carefully and contrast a) and b) with c) and d). The purpose of preparing solution d) in 1,2 dichloroethane is to show what the spectrum of the complex is in a non-co-ordinating solvent. Hint: Trans effect, and the pyridine is labile
- What is the valence electron count of the metal centre in the complexes [Co(CH3)(dmgH)2py], [Co(dmgH)2(MeOH)(py)] and [Co(dmgH)2py]? Hint: Use the 18e- rule.
References
- 'Hexol', Molecule of the Month, Sept 1997, via http://www.bris.ac.uk/Depts/Chemistry/MOTM/motm.htm
- 'Vitamin B12', Molecule of the Month, May 1997. via http://www.chm.bris.ac.uk/motm/vitaminb12/
- EMR Spectroscopy - all you ever wanted to know!
- JCAMP - and a method for viewing many types of spectra on the Web, see IUPAC JCAMP