Institute of Organic Chemistry, University of Innsbruck, A-6020 Innsbruck, Austria
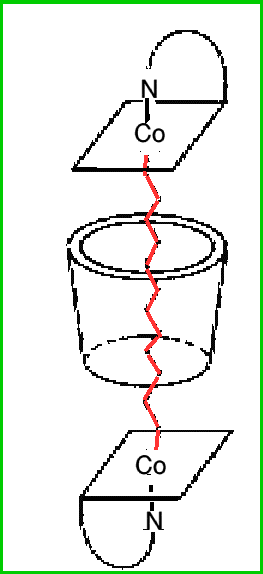
 In this intriguing molecule two
B12 units are linked via cobalt-carbon bonds across
a dodecamethylene, that is(CH2)12 chain.
In this intriguing molecule two
B12 units are linked via cobalt-carbon bonds across
a dodecamethylene, that is(CH2)12 chain.
In one form (to the left) this chain is surrounded by an alpha-cyclodextrin collar so that the cob(III)alamin units act as a pair of stoppers! In the other form (to the right) the alpha-cyclodextrin collar is omitted.
Both materials are soluble in water, but the collar shields the hydrocarbon chain from water molecules, allowing the chain to adopt a more relaxed conformation. With the alkyl-bridged dimer hydrophobic interactions with the water molecules cause a different conformation, and effectively the chain contracts, pulling the B12 units towards one another.
The material thus behaves a little like a "molecular spring", which can be "loaded" in one form (without the collar) and "contracted" in the other (with the collar).
The compound, whilst a curiosity in some ways, is of great interest for what it may tell about hydrophobic interactions, which are of course extremely important in biomolecular structures.