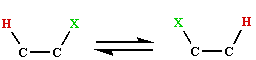 is believed
to take place by way of a radical mechanism, such rearrangements are very rare in
organic chemistry, indeed the only known instance is the Kharash-Urey reaction.
It is especially surprising that the reactions involve specific configuration changes
of the substrates. The evidence
for a radical mechanism comes principally from observations of EMR spectra for several of
the active enzyme systems. Thus if the dioldehydrase (holoenzyme) is treated
with the substrate analogue 1,2-propoanediol, a radical signal believed to be due to
the 5'-deoxyadenosyl radical,
together with a low spin Co(II) signal is formed within milliseconds, and persists
whilst there is substrate present {T.H.Finley, J.Valinsky, K.Sato, and R.H.Abeles,
J.Biol.Chem., 1972, 247, 4197}. Similar observations have been made for
dioldehydrase-substrate, glycerol-dehydrase, ethanol-ammonia lyase, and also the
ribonucleotide reductase system. Since it is necessary to rigorously exclude dioxygen
from any reaction involving free radicals, a plausible reaction mechanism {proposed
in da Silva and Williams seminal text}.
is believed
to take place by way of a radical mechanism, such rearrangements are very rare in
organic chemistry, indeed the only known instance is the Kharash-Urey reaction.
It is especially surprising that the reactions involve specific configuration changes
of the substrates. The evidence
for a radical mechanism comes principally from observations of EMR spectra for several of
the active enzyme systems. Thus if the dioldehydrase (holoenzyme) is treated
with the substrate analogue 1,2-propoanediol, a radical signal believed to be due to
the 5'-deoxyadenosyl radical,
together with a low spin Co(II) signal is formed within milliseconds, and persists
whilst there is substrate present {T.H.Finley, J.Valinsky, K.Sato, and R.H.Abeles,
J.Biol.Chem., 1972, 247, 4197}. Similar observations have been made for
dioldehydrase-substrate, glycerol-dehydrase, ethanol-ammonia lyase, and also the
ribonucleotide reductase system. Since it is necessary to rigorously exclude dioxygen
from any reaction involving free radicals, a plausible reaction mechanism {proposed
in da Silva and Williams seminal text}.
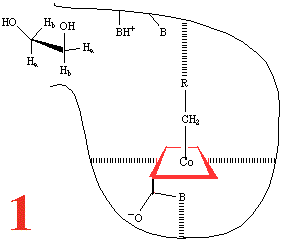
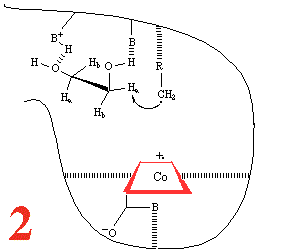 Step 1, and the substrate, 1,2-dihydroxyethane,
approaches the holoenzyme, and is about to plug the reaction 'bottle'.
Then (Step 2) the substrate binds
and the cobalt-carbon bond breaks homolytically to give the 5'-adenosyl
radical and a Co(II) corrin, anchored to the large coenzyme
(are the amide groups involved?) It has been
suggested that the cobalt-carbon bond, already a rather weak bond
and readily subject to photolysis if the holoenzyme is subjected
to visible light, is further
weakened by binding of the metalloenzyme to the large coenzyme.
Step 1, and the substrate, 1,2-dihydroxyethane,
approaches the holoenzyme, and is about to plug the reaction 'bottle'.
Then (Step 2) the substrate binds
and the cobalt-carbon bond breaks homolytically to give the 5'-adenosyl
radical and a Co(II) corrin, anchored to the large coenzyme
(are the amide groups involved?) It has been
suggested that the cobalt-carbon bond, already a rather weak bond
and readily subject to photolysis if the holoenzyme is subjected
to visible light, is further
weakened by binding of the metalloenzyme to the large coenzyme.
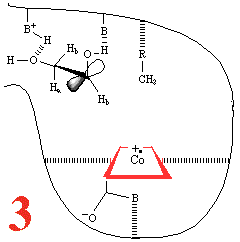
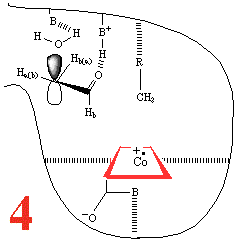 In Step 3,a hydrogen atom is abstracted from the
substrate by the 5'-deoxyadenosyl
radical, converting the dangling CH2 into a CH3
Then, Step 4, the enzyme base (B) and the acid
(BH+) cause assisted
beta-hydroxy fragmentation, that is loss of a water molecule.
In Step 3,a hydrogen atom is abstracted from the
substrate by the 5'-deoxyadenosyl
radical, converting the dangling CH2 into a CH3
Then, Step 4, the enzyme base (B) and the acid
(BH+) cause assisted
beta-hydroxy fragmentation, that is loss of a water molecule.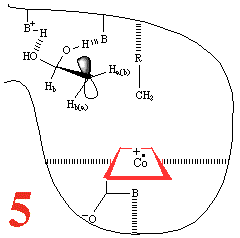
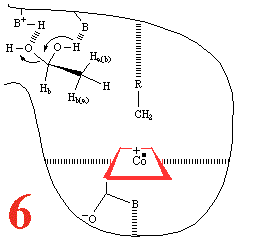 With Step 5, comes the re-addition of a water molecule,
resulting in a substrate reorientation
of C2 towards the 5'deoxyadenosyl methyl. There must be
competing C1 and C2 racemisation.
A hydrogen atom abstraction occurs, with competing C2 racemization.
The cycle is completed (Step 6), by substrate dehydration and release of ethanal and a water
molecule, so that the system can pick up another substrate molecule.
With Step 5, comes the re-addition of a water molecule,
resulting in a substrate reorientation
of C2 towards the 5'deoxyadenosyl methyl. There must be
competing C1 and C2 racemisation.
A hydrogen atom abstraction occurs, with competing C2 racemization.
The cycle is completed (Step 6), by substrate dehydration and release of ethanal and a water
molecule, so that the system can pick up another substrate molecule.
For the methyl transfer reactions involving CH3-B12, it is likely that Co(I) is involved, rather than a radical mechanism. More about this later!