Electron Ionisation (EI)
Electron Ionisation (often incorrectly called Electron Impact) is
generally considered to be the 'classical' method of analyte
ionisation. It is still routinely used today for the analysis of
low-mass, volatile, thermally stable organic compounds which are
difficult to ionise by other techniques. EI is especially useful when
coupled with gas chromatography (GC-MS).
Firstly the analyte must be vaporised. This is usually achieved by heating the probe tip containing a droplet of the analyte in solution. If the sample is thermally unstable, this will often be the first cause of sample fragmentation. Once in the gas-phase, the analyte passes into an EI chamber (see figure) where it interacts with a homogeneous beam of electrons typically at 70 electron volts energy. The electron beam is produced by a filament (rhenium or tungsten wire) and steered across the source chamber to the electron trap. A fixed magnet is placed, with opposite poles slightly off-axis, across the chamber to create a spiral in the electron beam. This is to increase the chance of interactions between the beam and the analyte gas. There are no actual collisions between analyte molecules and electrons, ionisation is caused by electron ejection from the analyte or by analyte decomposition. The scheme below shows some of the processes that can occur during the EI ionisation.
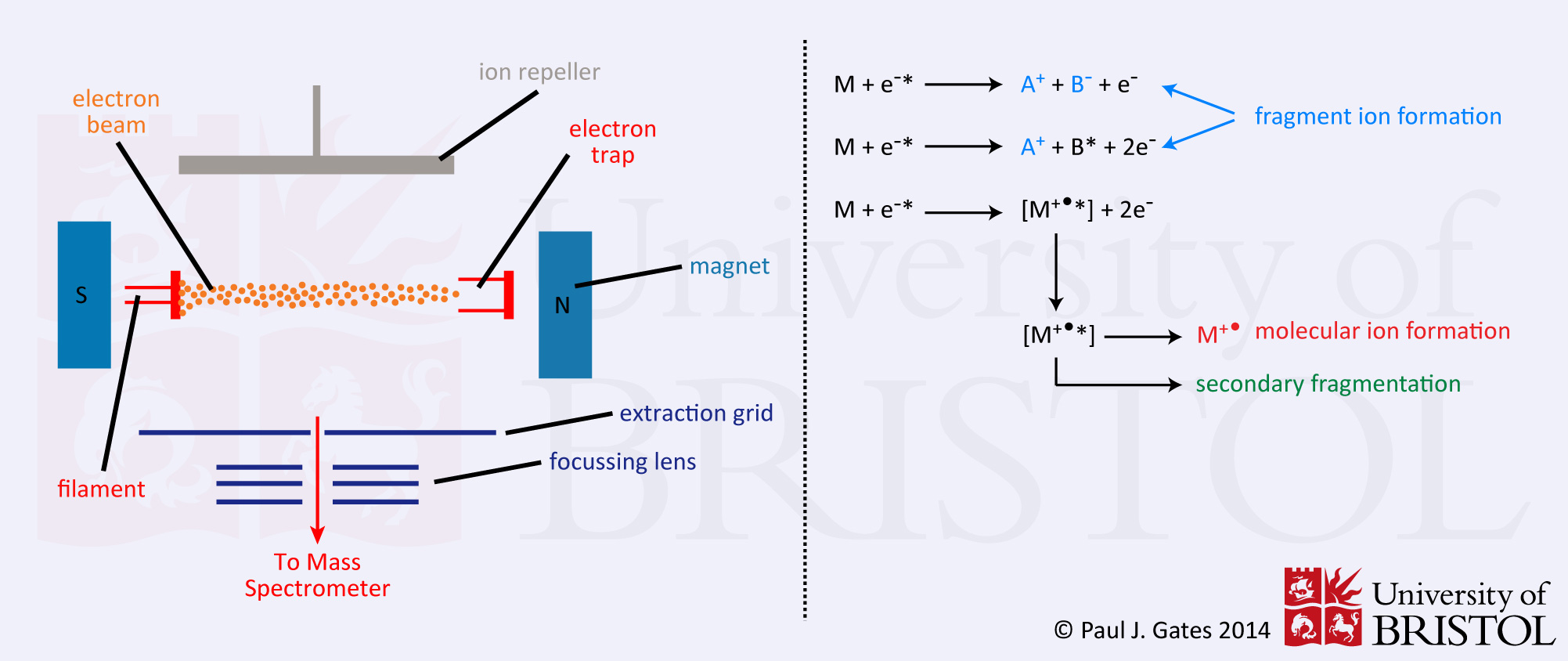
Consider the analyte molecule AB. The first two process that might occur are the direct result of energy transfer from the electron beam to the analyte, causing primary fragmentation and the second main cause of fragment ions in the spectrum. The third process is electron ejection from the analyte to create the energised radical ion. This can then either lose energy through 'ion cooling' and stabilisation (accounting for the radical molecular ion in the spectrum) or lose energy through secondary fragmentation - the third cause of fragment ions in the mass spectrum. These high levels of fragmentation in EI spectra often result in the the technique being termed a 'hard' method of ionisation. The harsh conditions required to volatilise some types of analyte and the high levels of residual energy possessed by the ions after ionisation result in the high amount of fragmentation observed in the mass spectrum often resulting in the complete absence of any intact molecular ion species.
Firstly the analyte must be vaporised. This is usually achieved by heating the probe tip containing a droplet of the analyte in solution. If the sample is thermally unstable, this will often be the first cause of sample fragmentation. Once in the gas-phase, the analyte passes into an EI chamber (see figure) where it interacts with a homogeneous beam of electrons typically at 70 electron volts energy. The electron beam is produced by a filament (rhenium or tungsten wire) and steered across the source chamber to the electron trap. A fixed magnet is placed, with opposite poles slightly off-axis, across the chamber to create a spiral in the electron beam. This is to increase the chance of interactions between the beam and the analyte gas. There are no actual collisions between analyte molecules and electrons, ionisation is caused by electron ejection from the analyte or by analyte decomposition. The scheme below shows some of the processes that can occur during the EI ionisation.

Consider the analyte molecule AB. The first two process that might occur are the direct result of energy transfer from the electron beam to the analyte, causing primary fragmentation and the second main cause of fragment ions in the spectrum. The third process is electron ejection from the analyte to create the energised radical ion. This can then either lose energy through 'ion cooling' and stabilisation (accounting for the radical molecular ion in the spectrum) or lose energy through secondary fragmentation - the third cause of fragment ions in the mass spectrum. These high levels of fragmentation in EI spectra often result in the the technique being termed a 'hard' method of ionisation. The harsh conditions required to volatilise some types of analyte and the high levels of residual energy possessed by the ions after ionisation result in the high amount of fragmentation observed in the mass spectrum often resulting in the complete absence of any intact molecular ion species.