Electrospray Ionisation (ESI)
The phenomena of electrospray has been known about for hundreds of
years, but is was not until the 1930's that its significance to
science was fully understood [1]. Some 30 years later, the pioneering
experiments by Malcom Dole et. al.
demonstrated the use of electrospray to ionise intact chemical species
and the technique of electrospray ionisation (ESI) was invented [2]. A
further 20 years elapsed until work in the laboratory of John Fenn
demonstrated for the first time the use of ESI for the ionisation of
high mass biologically important compounds and their subsequent
analysis by mass spectrometry [3]. This work was to win John Fenn a
share of the 2002 Nobel prize for chemistry - the 4th time a Nobel
prize has been awarded to mass spectrometry pioneers [4]. In the
original papers from the late 1980's, Fenn and his co-workers
successfully demonstrated the basic experimental principles and
methodologies of the ESI technique, including soft ionisation of
involatile and thermally labile compounds, multiple charging of
proteins and intact ionisation of complexes. ESI-MS is now a basic
tool used in probably every biological chemistry laboratory in the
world. The applications of ESI-MS are far too vast to enter into here,
but we recommend the following text book for further information [5].
Also, doing a quick 'Google' search on electrospray ionisation will
produce hundreds of matches.
The ESI source has undergone continued development since the earliest examples, but the general arrangement has remained basically the same (see figure 1). The analyte is introduced to the source in solution either from a syringe pump or as the eluent flow from liquid chromatography. Flow rates are typically of the order of 1µl/min. The analyte solution flow passes through the electrospray needle that has a high potential difference (with respect to the counter electrode) applied to it (typically in the range from 2.5 to 5 kV). This forces the spraying of charged droplets from the needle with a surface charge of the same polarity to the charge on the needle. The droplets are repelled from the needle towards the source sampling cone on the counter electrode (shown in blue). As the droplets traverse the space between the needle tip and the cone and solvent evaporation occurs. This is circled on the figure 1 and enlarged upon in figure 2.
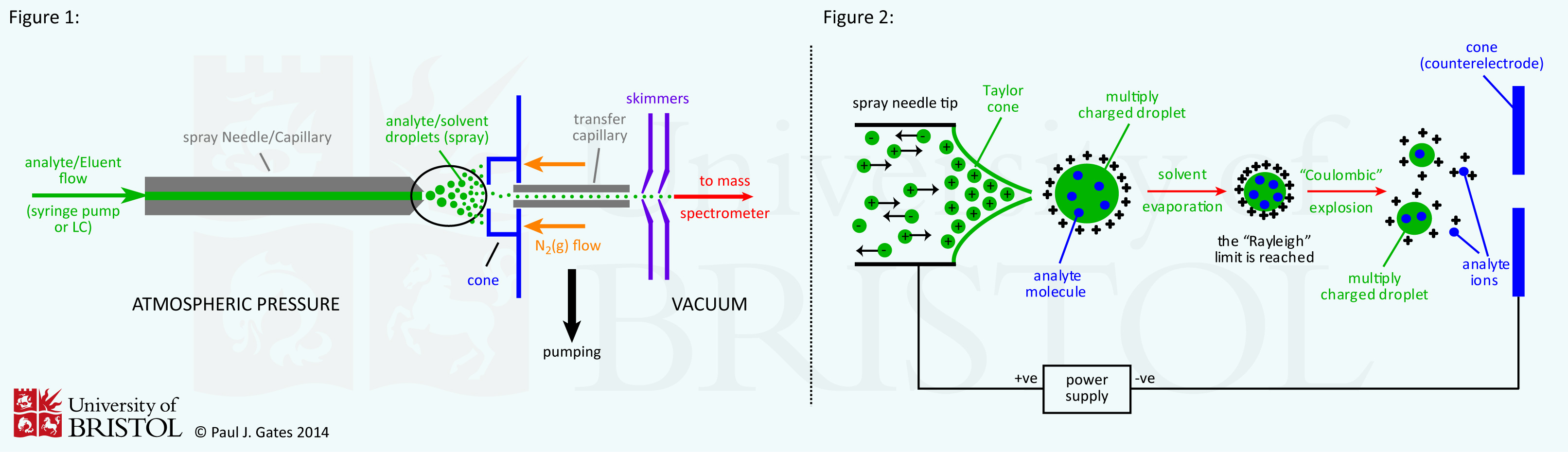
As the solvent evaporation occurs, the droplet shrinks until it
reaches the point that the surface tension can no longer sustain the
charge (the Rayleigh limit) at which point a "Coulombic explosion"
occurs and the droplet is ripped apart. This produces smaller droplets
that can repeat the process as well as naked charged analyte
molecules. These charged analyte molecules (they are not strictly
ions) can be singly or multiply charged. This is a very soft method of
ionisation as very little residual energy is retained by the analyte
upon ionisation. This is why ESI-MS is such an important technique in
biological studies where the analyst often requires that non-covalent
molecule-protein or protein-protein interactions are representatively
transferred into the gas-phase. The major disadvantage of the
technique is that very little (usually no) fragmentation is produced.
For structural elucidation studies, this leads to the requirement for
tandem mass spectrometry where the analyte molecules can be
fragmented.
The ESI source has undergone continued development since the earliest examples, but the general arrangement has remained basically the same (see figure 1). The analyte is introduced to the source in solution either from a syringe pump or as the eluent flow from liquid chromatography. Flow rates are typically of the order of 1µl/min. The analyte solution flow passes through the electrospray needle that has a high potential difference (with respect to the counter electrode) applied to it (typically in the range from 2.5 to 5 kV). This forces the spraying of charged droplets from the needle with a surface charge of the same polarity to the charge on the needle. The droplets are repelled from the needle towards the source sampling cone on the counter electrode (shown in blue). As the droplets traverse the space between the needle tip and the cone and solvent evaporation occurs. This is circled on the figure 1 and enlarged upon in figure 2.


One of the main problems with the mass
spectral analysis of proteins (and other macromolecules) has always
been that their masses fall outside the mass ranges of most mass
spectrometers. Before the development of ESI, the only real
practical ionisation method for the analysis of biological samples
was fast atom bombardment (FAB). But this technique produces a
predominance of singly charged ions and was best coupled to sector
instruments with mass ranges up to m/z
8 kDa at best. This severely limited analysis. The only way around
this was to digest the protein and analyse the digest mixture.
Although protein digestion is still an important technique for mass
spectrometry, it is now relatively easy to obtain direct mass
measurements of proteins by ESI-MS. The multiple charges (usually
protons) are statistically distributed upon the basic sites of the
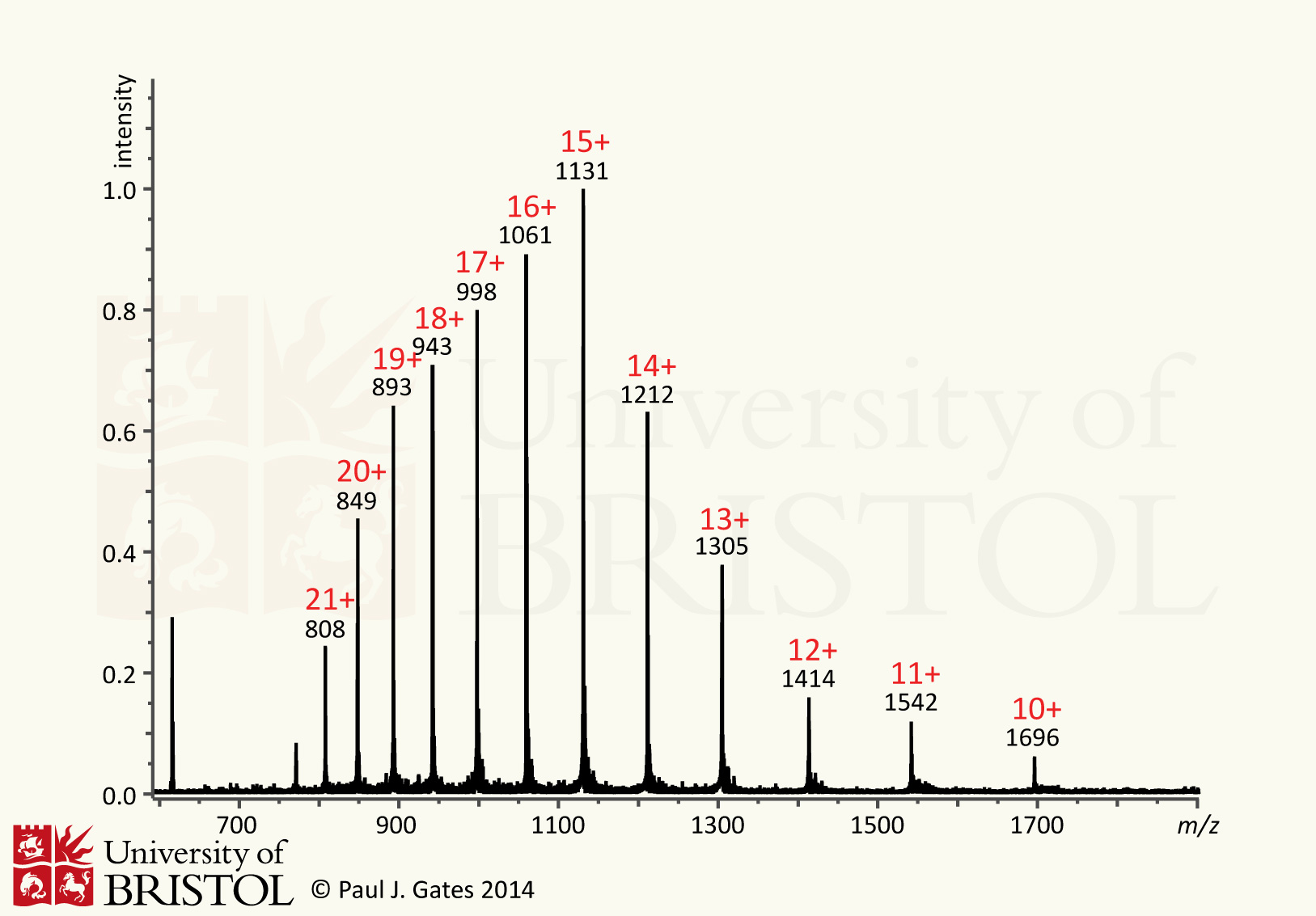
protein. Figure 3 shows a typical ESI mass spectrum of horse heart
myoglobin (16.95 kDa). The peaks observed are due to the multiple
charging, in this case the charges are roughly Gaussian distributed
around the +15 charge state (m/z
1131 = [M+H15]15+) ranging from +22 to +10.
The actual distribution of charges is dependent on a number of
factors, but most commonly the electrospray conditions can greatly
affect charge distribution as well as the gross structure of the
protein (i.e. the actual availability of the basic sites). Studies
of charge distribution are commonly used to make inferences about
the tertiary structure of the protein.
[2] M. Dole et. al., Journal of Chemical Physics, 49, 1968, p2240 and Journal of Chemical Physics, 50, 1970, p4977.
[3] J.B. Fenn et. al., Journal of Physical Chemistry, 88, 1984, p4451 and Science, 246, 1989, p64.
[4] J.B. Fenn, Agewandte Chemie - International Edition, 42, 2003, p3871.
[5] B.N.Pramanik, A.K. Ganguly and M.L. Gross, Applied Electrospray Mass Spectrometry - Volume 32 of the Practical Spectroscopy Series. Pub. Marcel Dekker, New York, 2002, ISBN: 0-8274-0618-8.
[6] W.J. Griffiths et. al., Biochemical Journal, 355, 2001, p545.
[7] S.J.Gaskell, Journal of Mass Spectrometry, 32, 1997, p677.
A good graduate student level
tutorial on ESI was published by Simon Gaskell in 1997 [7].
References:
[1] S. Chapman, Physical Review, 10, 1937, p184.[2] M. Dole et. al., Journal of Chemical Physics, 49, 1968, p2240 and Journal of Chemical Physics, 50, 1970, p4977.
[3] J.B. Fenn et. al., Journal of Physical Chemistry, 88, 1984, p4451 and Science, 246, 1989, p64.
[4] J.B. Fenn, Agewandte Chemie - International Edition, 42, 2003, p3871.
[5] B.N.Pramanik, A.K. Ganguly and M.L. Gross, Applied Electrospray Mass Spectrometry - Volume 32 of the Practical Spectroscopy Series. Pub. Marcel Dekker, New York, 2002, ISBN: 0-8274-0618-8.
[6] W.J. Griffiths et. al., Biochemical Journal, 355, 2001, p545.
[7] S.J.Gaskell, Journal of Mass Spectrometry, 32, 1997, p677.