Home Research Paul Wyatt People Publications Grants & Positions |
Research
Our
research divides broadly into
two areas –
Asymmetric
Synthetic Methods
We
look at novel ways to prepare single enantiomers. Although the
compounds themselves may be useful it is usually the tools we develop
to make them that are more important. These tools have intriguing
properties about them because of the symmetry they employ.
Although the investigation and subsequent application of some symmetry
elements has been thorough and successful, the study of properties of
molecules with higher symmetry has had little attention. Our
programme of research concerned with the investigation of the more
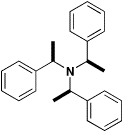
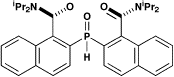
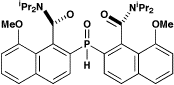
exotic symmetry elements and chirality. These include —
Centrosymmetry
Other
areas of research include –work ongoing...
C3 symmetry
Atropisomerism These 2-D TLC plates show how the compounds 1,2 and 3 interconvert (and 4, 5 and 6 do not interconvert) on the TLC timescale. In the first case the compounds have interconverted before the TLC is turned by 90° and run for the second time to give off-diagonal spots.
These compounds featured in the Tetrahedron Special Issue about atropisomerism – P. Wyatt, P. Hooper and F. Sternfeld, Tetrahedron, 2004, 60, 4549-4558 New
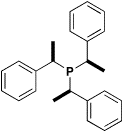
ways to reduce phosphine oxides
This work was conducted by undergraduates who showed that not only was aluminium hydride (AlH3) excellent for reducing phosphine oxides to phosphines, it was also a highly chemoselective reagent. A. Bootle-Wilbraham, S. Head, J. Longstaff and P. Wyatt, Tetrahedron Lett., 1999, 40, 5267-5270 Chiral Heterocycles A double lithiation strategy was employed as our main way to make novel sulfur containing rings. Another novel strategy made use of a double Pummerer rearrangement to make hetereocycles containing a disulfide link. P.
Wyatt and A. Hudson, Organic and
Biomolecular Chemistry, 2004,
2,
1528-1530
A. Hudson, A. G. Orpen, H. Phetmung and P. Wyatt, Tetrahedron Lett., 1999, 40, 813-816 Photoactive
Surface Science
We collaborate on projects with Prof. Julian Eastoe in Physical Chemistry investigating photo-active surfactants. Modfiying the behaviour of a detergent with light is the cleanest, 'greenest', way to achieve that modification. Methods have previously used other chemical agents (e.g. salts) which have their own behaviour and add to chemical consumption. If shining light on a droplet causes it to fall then the applications are manifold (precise delivery of ink droplets for example). This work made the front cover of Langmuir, the leading American journal on surface science. Shining
light on a droplet changes the surface tension.
 This is also very evident by the contact angle that is formed with a surface (67.5° falls to 52.5°) 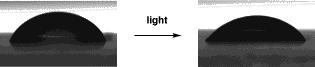 J. Eastoe, M. Sanchez-Domingez, P. Wyatt, A. Beeby and R. K. Heenan, Langmuir, 2002, 18, 7837-7844 |