Other cyclic and polycyclic hydrocarbon molecules are also found
experimentally to be similarly stable (i.e. to have large resonance energies).
We can plot of the experimental resonance energy against the
predictions of Hückel Theory (you’ll calculate resonance energies for some of these
molecules, e.g. naphthalene, in the Theoretical Chemistry Class).

This plot gives b = 66.7 kJ/mol, slightly different from
the value we find for benzene itself.
·
The fact that we get a good correlation with experimental
values shows that Hückel Theory can give good predictions about molecular
properties (in this case stability).
Actually, we should probably allow for the fact that the
hypothetical, non-delocalized cyclohexatriene would not have the same geometry
as benzene (in which all the C-C bonds have the same length), because it would
consist of alternating double and single bonds
To estimate the delocalization energy, we should include the energy
penalty for distorting cyclohexatriene to a form in which all the bonds have
the same length:
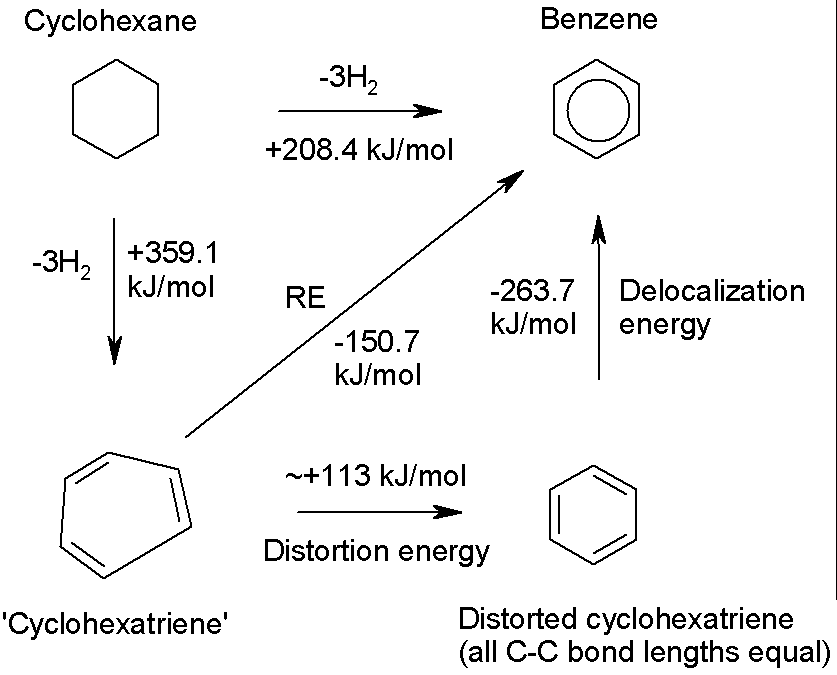
RE: ‘resonance energy’ found from experimental thermodynamic measurements. The distortion energy has been estimated from calculations.
From the cycle above, the corrected value
of the delocalization energy of benzene is –263.7 kJ/mol. This gives b as –131.85 kJ/mol.
It is important to remember that the
delocalization energy is one reason why benzene is stable, but the fact that
its hexagonal shape allows s-bonds to be formed with no strain also
contributes to its stability.