p electron atomic
charge
The coefficients in the molecular orbitals reflect the charge
distribution of an electron in that orbital.
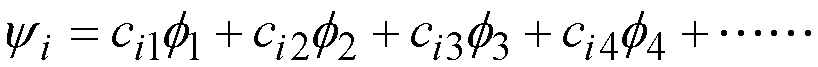
yi: Molecular orbital number ‘i’
f1: atomic (p) orbital on
atom 1
f1: atomic (p) orbital on
atom 1, etc.
In the coefficients cia
i -----
is the molecular orbital number
a ----- is the number of the atom and of
corresponding atomic p-p orbital.
If there is an electron (just 1) in molecular orbital yi the charge
distribution corresponding to this electron is given by:
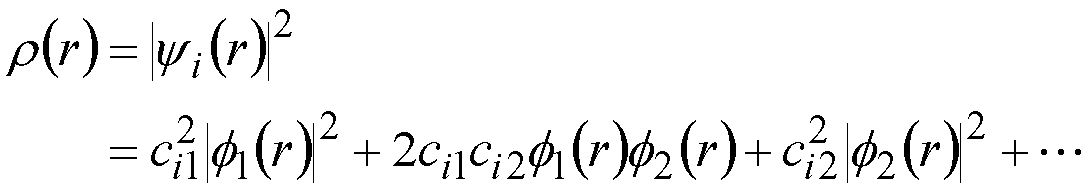
r(r): Charge distribution (electron
density) -- this is a function of position r -- arises from 1 electron
in M.O. “i”
The electron density is a function of the coordinates. Its value changes with position in space.
In the example above, ci12
is a measure of the amount of charge on atom 1 - arising from 1 electron
in MO i.
2ci1ci2 is a measure
of the charge shared between atoms 1 and 2.
p electron
charge on atom a (qa):
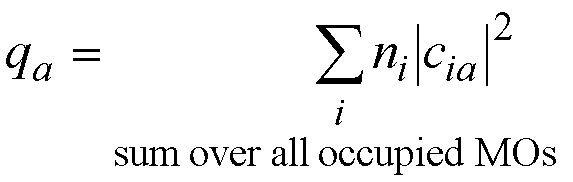
ni is the number of
electrons in MO i.
If the occupied molecular orbitals all
have 2 electrons in them then ni=2 and the equation for the
atomic charge becomes;
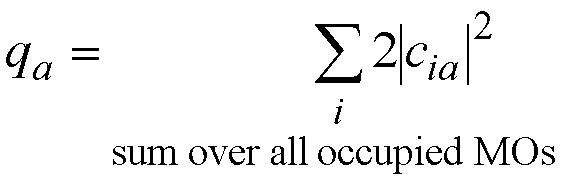
As you will see in the accompanying Theoretical Chemistry Class,
the charges from Hückel Theory predict so-called ‘non-alternant’ hydrocarbons
to be polar (have a dipole moment), while ‘alternant’ hydrocarbons are not, in
agreement with experiment.