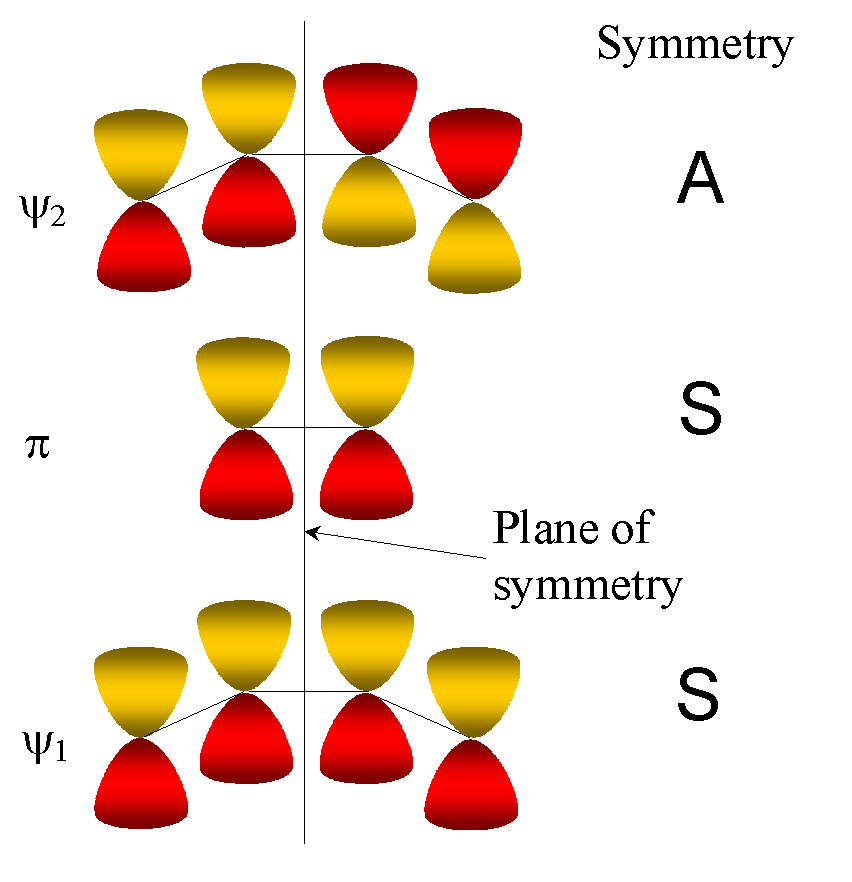
Analysing the symmetry of
the reactant molecular orbitals
·
We now follow the same procedure for the basic Diels-Alder
reaction as we did for the cycloaddition of 2 ethene molecules.
·
We consider the MO diagram of the active MOs in the reactants and
in the products and draw a correlation diagram -- with conservation
of orbital symmetry.
Molecular
orbitals of reactants
Lowest 3 MOs of reactants (butadiene +ethene):