Pericyclic reactions and electrocyclic reactions
·
A pericylic reaction is one in which concerted
reorganization of bonding occurs throughout a cyclic array of continuously
bonded atoms (e.g. Cope rearrangement, Diels-Alder reaction)
·
A subclass of pericyclic reactions is that of electrocyclic
reactions in which a ring is formed by the formation of a single, s bond between the ends of an acyclic conjugated p system (or the reverse reaction)
An example of an electrocyclic reaction is the ring opening of
cyclobutene to give 1,3-butadiene:
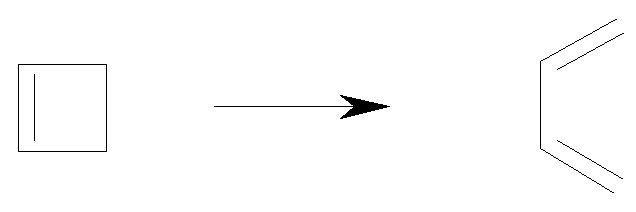
This ring opening reaction is stereospecific: it is conrotatory.
This means that the rotation of the two terminal groups is in
the same sense - both clockwise or both anti-clockwise.
Examples:
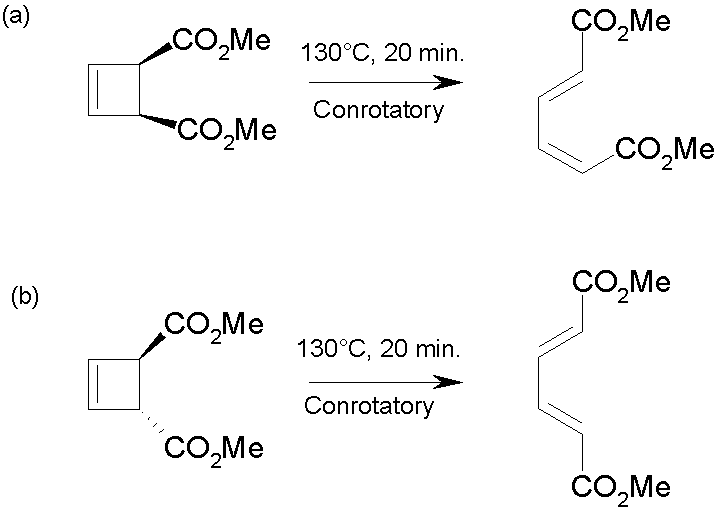
·
Ring opening is favoured by thermodynamics, but the
stereochemistry is not determined by thermodynamics
·
For example, in (a) the more strained, thermodynamically less stable
product (with one double bond cis) is
formed.