Charge transfer interactions
One important interaction
that can be explained by the frontier orbital picture is the formation of a
complex between molecules involving charge transfer from one molecule to the
other.
Some of the charge of the ‘donor’ molecule is transferred to the
‘acceptor’ when the complex is formed:
D + A
→ DA
Remember that the HOMO is the easiest orbital in a molecule to
remove an electron from, while the LUMO is the easiest to which to add one.
In the donor-acceptor complex, electrons that had been solely
localized on the donor (in the HOMO) now occupy an orbital which involves the
acceptor as well:
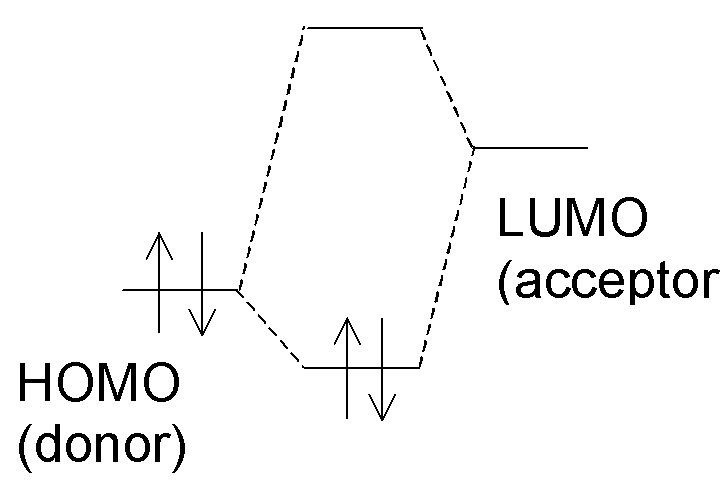
The net result is
that electron density increases on the acceptor and decreases on the donor,
leading to a partial negative charge on the acceptor and partial positive
charge on the donor: Dd+Ad–
Charge transfer may be important in binding of some biological ligands to their receptors, for example:
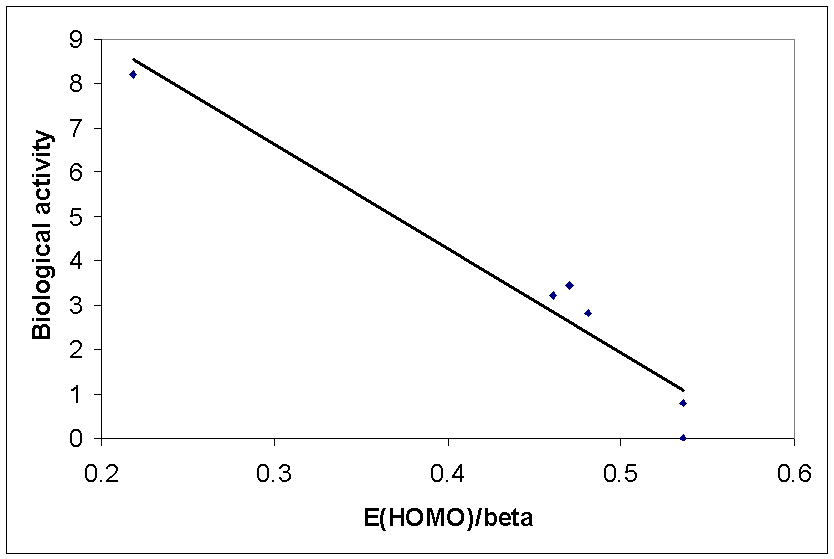
Correlation of
Hückel HOMO energy (in units of b, i.e.
negative) with hallucinogenic potency for some drugs: better donors are more
active.
This correlation
does not prove that charge transfer is involved in binding or activity, but
correlation of calculated and experimental properties can help pharmaceutical
development and testing.