 Low Temperature Diamond CVD using CO2/CH4 gas mixtures
Low Temperature Diamond CVD using CO2/CH4 gas mixtures
The now well established conditions for diamond growth include the use of substrate temperatures >>700°C and a carbon-containing precursor gas diluted in excess hydrogen (typically <5% CH4 in H2). A major goal in the field of diamond CVD is the lowering of substrate temperatures required for growth, as this could permit the use of a much wider range of substrate materials of industrial importance, such as aluminum, GaAs, nickel and steel.
Many gas mixtures containing varying ratios of O, C and H have been investigated in the search for a viable low temperature diamond deposition process. Work within our group has centred on microwave Plasma CVD diamond deposition using CO2/CH4 gas mixtures. Investigations of both low temperature growth and gas phase plasma chemistry have been carried out, results of which are discussed below.
Low Temperature Diamond Growth
It has been found that the highest quality diamond is obtained using the mixture 50% CH4/50% CO2. This gas mixture has also been found to allow diamond growth at substrate temperatures <500°C although a clear reduction in the crystallinity of the films with reduced temperature can be seen.
Lowering substrate temperature also has the effect of lowering film growth rate . Using these growth rate data an Arrhenius plot can be produced which yields an overall activation energy for diamond deposition of 28 kJ mol-1. This is lower than previously obtained for CH4/H2 systems and hints at a different surface chemistry in the two systems.
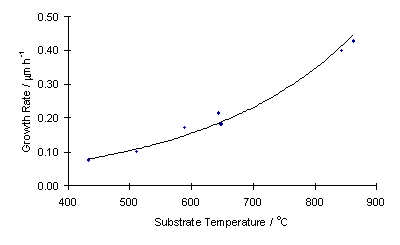 |
| Growth rate vs substrate temperature |
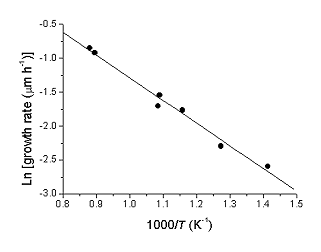 |
| Arrhenius plot of growth rate data |
CO2/CH4 plasma chemistry
Studies have shown that CO2/CH4 mixtures containing >50% CO2 result in no film deposition, 50%CO2/50%CH4 gives optimum diamond growth whilst increasing %CH4 above 50% produces increasingly graphitic material. In order to explain these observations molecular beam mass spectrometry was used to make measurements of plasma species concentrations over a wide range of mixing ratios (0-80% CH4). Computer simulation of these results was then carried out using the CHEMKIN II computer package thus allowing the important plasma reactions to be identified.
- CO2 + CH4 = 2CO + 2H2
- CO2 + H2 = CO + H2O
- H2O + C2H2 = CH4 + CO
Reaction 3 is important because it leads to a peak in CH4 (and therefore CH3) concentration at 50% input CH4. CH3 is believed to be the growth species in diamond CVD using CH4/H2 gas mixtures. The peak in CH3 concentrations at the same composition as the optimum diamond growth is obtained suggests that CH3 is also the diamond growth species for CO2/CH4 gas mixtures.
The different activation energy obtained for 50%CO2/50%CH4 compared with CH4/H2 gas mixtures suggests a difference in the growth mechanism. This could be due to a difference in the surface termination of the growing diamond surface which is thought to be performed by H in CH4/H2 systems. Given the relatively high concentration of CO present at 50%CO2/50%CH4 it seems reasonable that at least some of the diamond surface could be terminated with CO (or HCO) in this system.
For more information, please see this reference.
