A1. Ablation plume properties arising from ArF PLD of aluminium and copper
The properties of plumes arising from ablation of metallic targets has been investigated briefly during this thesis work. The main reason for this study was to provide a comparison with the characteristics of carbon and ZnO ablation plumes, which were subjected to a more in depth analysis (see previous chapters). The results illustrate interesting universal properties of ablation plumes, namely their high degree of ionisation and the high kinetic energy distribution of the ions. The results also support previously introduced concepts of heating of the ablation plume by laser-plasma interaction and acceleration of the ions so created by a fast 'non-thermal' electron distribution. Study of the ablation plumes not only provides insight into the fundamental principles, but also gives a reference for the deposition of films from these ablation fluxes. The study of the ablation characteristics of aluminium and copper is a necessary prerequisite background for understanding of the production of, respectively, aluminium nitride (AlN)[1] films and Cu-Co Giant Magneto-Resistant (GMR)[2] films by pulsed laser deposition.
A1.1. Time integrated OES
The fluence dependence of the time integrated OES following 193 nm pulsed laser ablation of copper and aluminium in vacuum has been investigated at a distance of 5 mm from the target along the target surface normal. Spectra obtained for both species for an incident fluence of 20 J/cm2 are given in Figure 1.1.
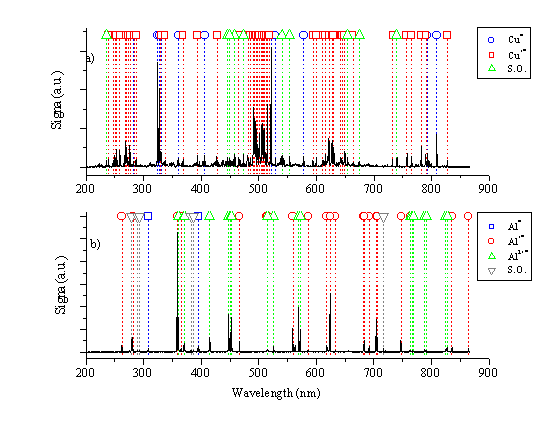
Figure 1.1: Wavelength dispersed, time integrated OES for (a) copper and (b) aluminium monitored 5 mm from the target for a fluence of 20 J/cm2. The combs identify the various emitting species within the plume. SO stands for second order. Emission line wavelengths are obtained from reference [3].
The wavelength dispersed, time integrated OES for aluminium consists of emission from neutral Al atoms, and Al+ and Al2+ cation species in the plume. The wavelength dispersed, time integrated OES for copper is much richer than the optical emission spectra for other species (e.g. Al, Zn), but all of the emission lines can still be attributed emissions from Cu neutral and Cu+ ionic species in the plume.
The fluence dependence of the different emitting species contained within the two plumes at a distance of 5 mm is shown in Figure 1.2. As one can see, the dependence of the signal on the fluence follows a linear relationship for the neutral atomic emission lines and a higher order relationship for the ionic emissions. The observed fluence dependence of the Al+ and Cu+ emissions can both be fitted to second order polynomial relationships, while the fluence dependence of the Al2+ emission is best represented by a third order polynomial fitting function. Comparing the onset fluence for creation of Cu and Al species shows very different behaviour for the different species. The onset for Cu+ emission can be approximated from the graph to be around 2.5 J/cm2 while the onset for Al+ and even Al++ emission appear to be close to 0 J/cm2. The Al neutral emission line, in contrast, seems to show onset behaviour but this may be more a reflection of the fact that the Al neutral lines are very weak spectral features (see Figure 1.1) and the emission intensities are close to the detection limit at low fluences.
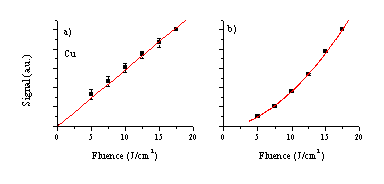
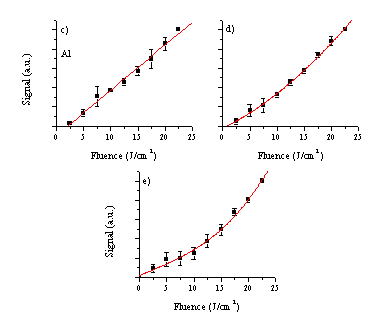
Figure 1.2: Measured fluence dependences for the various different emitting species, namely: (a) Cu*, (b) Cu+*, (c) Al*, (d) Al+* and (e) Al2+*.
The functional form of the fluence dependent relative emission intensities of the various different species are very comparable to the curves observed for ZnO. The neutral emission is well approximated by a linear relationship, while the functional forms of the ion emissions can be fitted by higher order polynomials. These observations can be explained in terms of the formation mechanisms for the species responsible for the various emissions. As has been discussed already, at length, in former chapters, the emission arising from an atom or ion in the plume is correlated with the existence of Rydberg states of this species in the plume. More importantly, these Rydberg states are products of recombination reactions. Thus the observed Mn+* (M = metal) emission is evidence for the existence of the parent M(n+1)+* ion in the plume, and the behaviour of these emitting species closely follows the behaviour of the precursor ions in the plume. The neutral emission, which we attribute to nascent singly charged ion formation in the plume, can readily form during the ablation process by interaction of the laser light with the ejected neutrals (preferentially via MPI). Doubly (and higher) ionised species will most probably be created by interaction of the laser light with the ions (preferentially via electron-ion IBE). In this discussion it is assumed that neutral-electron IBE is negligible compared to MPI as a route to ionising the neutral atoms. The observed higher order fluence dependence of the ionic emission lines is explicable by this mechanism since IBE is a non-linear process and the density of ions created by this mechanism, and thus the amount of emission, will scale non-linearly with the fluence. The fluence onset behaviour of the Cu ion emission can than also be explained, since the IBE process needs a sufficient ion fraction in the plume to become a significant process. The Al cation emission appears not to show this threshold behaviour. This will need to be explained more carefully considering more specifically the absorption process in this material.
Comparing the ionisation potentials (IP) of the two studied materials can provide some further insight into the anomalous behaviour for Al. The ionisation energies of the Cu and Al metal atoms and ions (up to charge 4) are given in Table 1.1.
|
|
Al |
Cu |
|
X+ |
5.99 |
7.73 |
|
X2+ |
24.81 |
28.02 |
|
X3+ |
53.26 |
64.86 |
|
X4+ |
173.25 |
122.23 |
Table 1.1: Energies (in eV) necessary to create the ions from the ground state neutral for copper and aluminium.
Important to note is that the energy to create an Al+ ion is lower than the energy of the laser photons (ArF = 6.4 eV), while the photon energy is lower than the ionisation potential of copper. This implies that Al+ ions can be formed by a one photon process. This is different to any of the previously studied materials, which always involved at least a two photon process to create an ion. As a consequence, an aluminium atom will be much more easily ionised by an ArF laser photon. A second consequence is that electron-ion IBE will gain in importance as an ionisation mechanism at low fluences.
A1.2. Faraday Cup measurements
TOF transients of the ion current were recorded for both copper and aluminium using the Faraday Cup assembly. The recorded TOF profiles, together with their converted velocity distributions, are given in Figure 1.3.
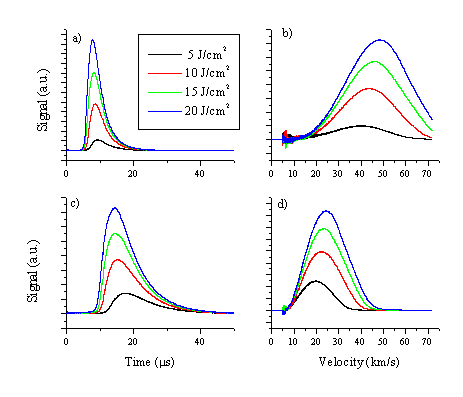
Figure 1.3: TOF profiles of the charged particles, measured with the Faraday Cup assembly, measured at the surface normal and a distance of 421.7 mm, and converted velocity distributions for Al ((a) - (b)), and Cu ((c) - (d)) for different fluences.
The fluence dependency of the velocity, and the kinetic energy, of the total ion yield for each species (Cu and Al) was determined from TOF profiles recorded at various incident pulse energies. Results are given in Figure 1.4. For calculating the kinetic energy, it was assumed that all detected charged particles are monatomic. This is probably a reasonable assumption given that the wavelength dispersed OES shows no evidence of any diatomic or larger emitting species (see also Chapter 4-5). Figure 1.4 presents a comparison of the mean ion kinetic energy distributions from Cu and Al ablation at 193 nm, in vacuum, as a function of incident fluence. The mean kinetic energy of both sets of metallic ions is higher than in the case of carbon. In addition, the mean kinetic energy of the aluminium ions is much higher than that of the copper ions. This supports the statement that laser plume heating mechanism is very effective in the case of Al ablation and is an important mechanism for coupling the energy of the laser photons with the kinetic energy of the emitted particles (in this case ions). The ionic products of the vacuum UV laser ablation of Al (at l=355 nm) and Cu (at l=351 nm) have been studied by both experimentally[4],[5] and theoretically[6] by Amuruso et al. In both cases the study revealed important laser-plasma interactions at the reported wavelength, actually, the tail-off at higher fluences, also visible in Figure 1.4, has been solely attributed to laser-plasma interactions. The velocity distribution for aluminium species has been studied in more detail, via OES and Langmuir probe measurements. Results of these studies are reported in the next paragraphs.
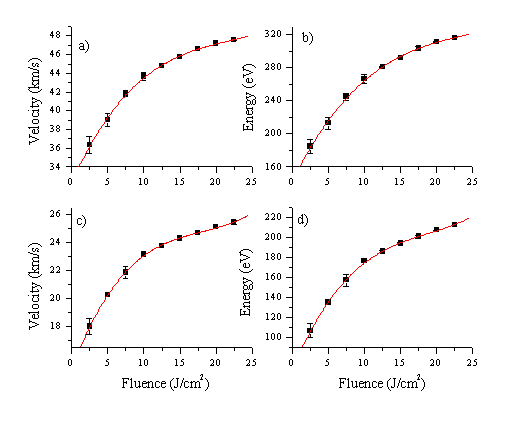
Figure 1.4: Fluence dependency of the velocity and the kinetic energy of the charged particles, measured with the Faraday Cup assembly, for aluminium ions ((a)-(b)) and copper ions ((c)-(d)).
A1.3. Time differentiated OES for aluminium
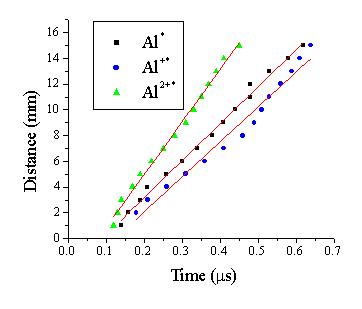
The time differentiated OES resulting from 193 nm pulsed laser ablation of Al was studied by monitoring three different emission lines at a fluence of 20 J/cm2, namely the 277.6 nm line for Al* neutral emission, the 335.1 nm line for Al+* emission and the 448.0 nm line for the Al2+* dication emission.3 Representative TOF transients for each of these emission lines measured at a distance of 7 cm from the target is given in Figure 1.5. This figure shows that the emitting Al* neutral and Al+* cations propagate with similar velocities while the emitting Al2+* species propagate faster.
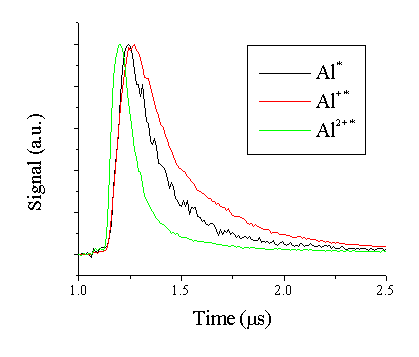
Figure 1.5: TOF transients for three emission lines, measured with optical emission spectrscopy, 7 mm from the target.
Figure 1.6: Mean velocity of emitting species in the ablation plume resulting from pulsed laser ablation of an Al target at 193 nm in vacuum, at the fluence of 20 J/cm2.
Table 1.2: Mean velocity of the three components of the emission deduced from the time versus distance plots such as those shown in Figure 1.5.
A1.4. Langmuir Probe transients for aluminium
The electron current from the ablation plume arising from the 193 nm ablation of aluminium in vacuum has also been studied, using the Langmuir probe assembly. The TOF transients recorded with the Langmuir probe positioned 3 cm from the target along the target surface normal are shown in Figure 1.7. The TOF profiles show a fluence dependent double peak behaviour. The early time peak in the TOF transient increases in intensity relative to the later peak as the incident fluence is increased.
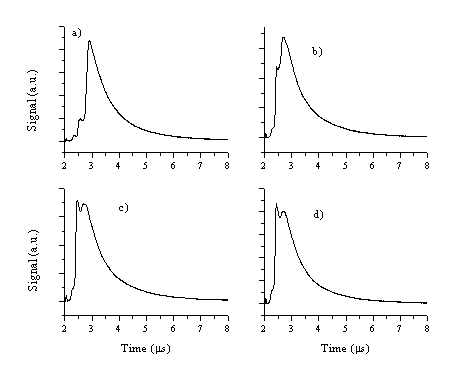
Figure 1.7: TOF transients recorded with the Langmuir probes at 3 cm from the target, the applied fluences are (a) 5 J/cm2, (b) 10 J/cm2, (c) 15 J/cm2 and (d) 20 J/cm2. The spectra are taken with a 2 ms delay time.
The mean velocity can be deduced from the TOF transients: and the results are summarised in Table 1.3.
|
Fluence (J/cm2) |
Velocity (km/s) |
|
5 |
21.0 ± 0.8 |
|
10 |
26.7 ± 0.4 |
|
15 |
27.9 ± 0.1 |
|
20 |
28.8 ± 0.4 |
Table 1.3: The mean velocity of the electrons monitored by a Langmuir Probe positioned 3 cm from the Al target, as a function of fluence.
The mean velocity of the distribution rises as a consequence of the higher fraction of the fast component, but the mean velocity of the total electron signal is still slower with that of the ions derived from the Faraday Cup transients. To investigate the possibility of an acceleration of the ions and electrons (like in references [7]-[9]), the electron current was also measured at a distance of 7 cm from the target and at the fluence of 20 J/cm2. The obtained TOF is given in Figure 1.8 with its conversion into a velocity distribution.
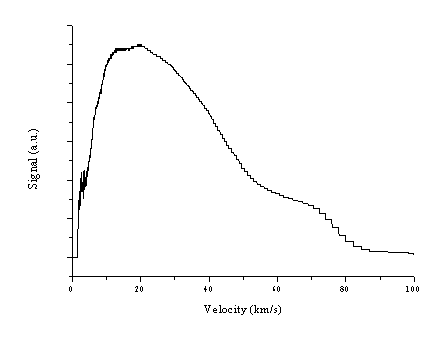
Figure 1.8: Velocity distribution of the electrons, measured by a LP at a distance of 7 cm from the target, for 193 nm Al ablation at a fluence of 20 J/cm2.
The derived mean velocity is 36.5 ± 0.5 km/s, significantly larger than the mean velocity derived at the distance of 3 cm, but still smaller than the ion velocity derived from the Faraday Cup transients. The recorded data suggest that both the electrons and the ions in the ablation plume undergo significant acceleration during their flight through vacuum. The acceleration is believed to have the same origin as the acceleration observed in ZnO, namely a hot 'non-thermal' electron distribution, which induces a bipolar expansion in the plume.
A1.5. Conclusion
The ablation of copper and aluminium at 193 nm, in vacuum, using focused nanosecond laser pulses was briefly studied. The OES spectra showed the presence of highly ionised plumes, and the ionisation could be attributed to combined effects of MPI and IBE. The higher degree of ionisation in the Al ablation plume is likely due to the fact that one ArF laser photon carries sufficient energy to ionise all neutral Al atoms, even in the ground state. Faraday Cup measurements revealed that the ionic component of the plumes propagate with very high kinetic energies (~ 200 eV for Cu and ~ 300 eV for Al). The development of such high kinetic energies has been investigated in the case of Al ablation via time resolved OES and Langmuir probe studies also. The Langmuir probes reveal the presence of a non-thermal electron component, which accelerates the plume, as in the case of ZnO (Chapter 5). Comparison between the Langmuir probes and the Faraday Cup measurements reveal an additional 40% increase in centre of mass expansion velocity of the ions as the plume expands from 30 to 421.7 mm from the target.