7. Results for H/C/O/S Systems
7.1. Introduction
This chapter builds on the work presented in the previous two chapters and presents the results of MWCVD diamond growth experiments using H2S additions to 51%CH4/49%CO2 (x=0‑0.5%) gas mixtures. Thus, the effect of lowering Tsub and the use of O containing gas mixtures on the growth of S-doped CVD diamond films can be investigated. OES and MBMS diagnostics have been used to investigate the gas-phase chemistry of such systems. The results are correlated with those of SENKIN computer simulations. The degree of S‑incorporation into the deposited films is discussed with reference to the parameter Fs (as introduced in Section 6.8).
7.2. Diamond Deposition From H2S/51%CH4/49%CO2 Gas Mixtures
MWCVD experiments were undertaken in which films were deposited using xH2S/51%CH4/49%CO2 (x=0-0.5%) gas mixtures with applied microwave power of 1 kW and pressure of 40 Torr. Deposition was undertaken at substrate temperatures of 620 and 900°C (as discussed in Section 3.2.9). Diamond films were analysed by SEM, LRS, four-point probe and XPS, as discussed below.
7.2.1. Film Crystallinity
Deposited films were examined by SEM in order to investigate the effect on film crystallinity and facet size of H2S addition to a 51%CH4/49%CO2 gas mixture. Figure 7.1 presents SEM images of diamond films grown at substrate temperatures of (a)-(c) 620°C and (d)-(f) 900°C, at various H2S additions. The film grown at the lower Tsub, using an H2S addition of 100 ppm (Fig. 7.1(a)) exhibits a continuous coating of predominantly triangular facets ~5 mm in size. Increasing H2S input levels to 2000 ppm decreases the facet size to < 2.5 mm (Fig. 7.1(b)), while 5000 ppm H2S additions produce a film primarily composed of small (< 1 mm) facets (Fig. 7.1(c)) with the occasional larger crystallite. In contrast, there is little change in the surface morphology of films grown at Tsub = 900°C for H2S additions of 100, 2000 and 5000 ppm, as illustrated by Figures 7.1(d), (e) and (f), respectively. Here, all three films are continuous and display crystalline facets ~5 mm in size.
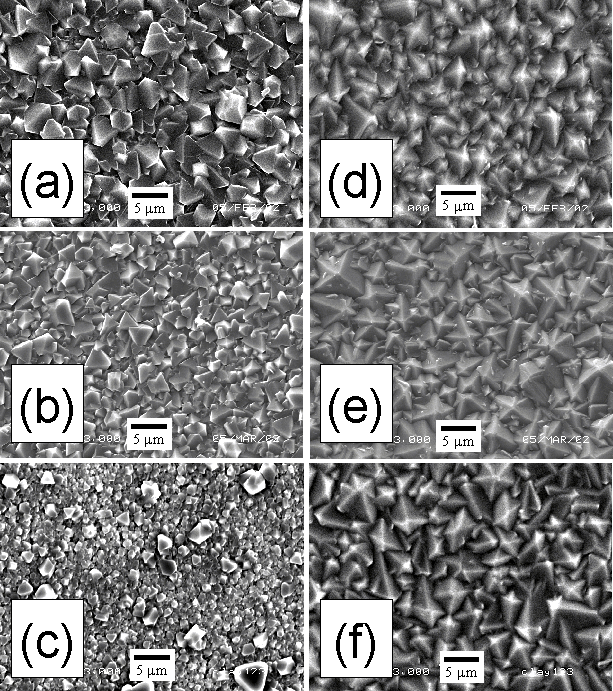
Figure
7.1. SEM micrographs for films grown using 51%CH4/49%CO2
gas mixtures and Tsub of 620°C with H2S
additions of (a) 100, (b) 2000 and (c) 5000 ppm, and Tsub = 900°C
with H2S additions of (d) 100, (e) 2000 and (f) 5000 ppm in a
MW plasma reactor. Conditions: total
gas flow 200 sccm, growth time 8 h, pressure 20 Torr, 1 kW
applied microwave power.
7.2.2. Film Growth Rate
Figure 7.2 shows that the growth rates of films grown at Tsub = 900°C are approximately one order of magnitude greater than for those grown at 620°C. However, despite this difference, the observed growth rate trend, i.e. a fall of ~66% when H2S addition is increased from 0 to 5000 ppm, is similar for both temperatures.
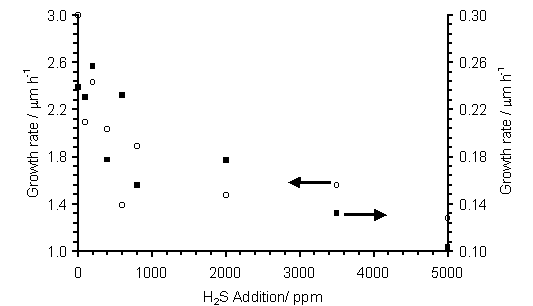
Figure 7.2. Film growth rate
(measured by cross-sectional SEM), versus H2S addition for films
grown in H2S/51%CH4/49%CO2 gas mixtures at two
substrate temperatures. Key: (■) Tsub = 620°C, (о) Tsub = 900°C, with other process
conditions as for Fig.7.1. Both data
sets show a ~66% reduction in growth rate over the range of H2S
addition shown.
7.2.3. LRS Analysis of Film Quality
Raman spectra of diamond films grown at an H2S addition of 5000 ppm at (a) 620°C and (b) 900°C are presented in Figures 7.3(a) and (b). Both spectra can be fitted using a quadratic base line, two Gaussian peaks centred at ~1332 cm-1 (sharp diamond Raman line and a broader D band), a peak at ~1530 cm-1 (the G band) and a broad band at ~1450 cm-1 (polyacetylene [[1]] or a diamond precursor [[2]]). However, a number of additional features are evident in the spectrum of the sample grown at the lower Tsub, including the presence of peaks at ~1172 cm-1 (nanophase diamond [[3]] or polyacetylene present in grain boundaries [1]) and 876, 967 and 1088 cm-1. These additional peaks may correspond with nanoscale sp3 phases resulting from the small sized crystallites grown at this low Tsub.
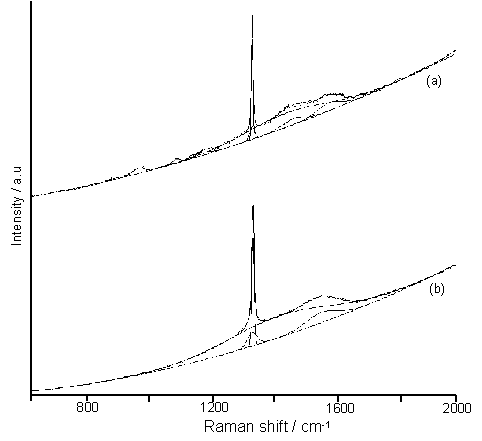
Figure 7.3. Laser Raman
spectra of films deposited using 5000 ppm H2S addition to 51%CH4/49%CO2
gas mixtures for (a) Tsub = 620°C and (b) Tsub = 900°C. Each spectrum includes a fitted quadratic baseline and Gaussian
line shape curve fits.
In order to gain a measure of film quality (i.e. the ratio of sp3:sp2 carbon bonding) the quality factor, Q (as discussed in Section 2.3) is presented in Figure 3(c). Somewhat surprisingly, Q for films grown at the lower Tsub is generally slightly higher than those deposited at 900°C. Although the scatter is considerable it is apparent that, in both cases there is a slight fall in film quality with increased H2S addition for both sets of data.
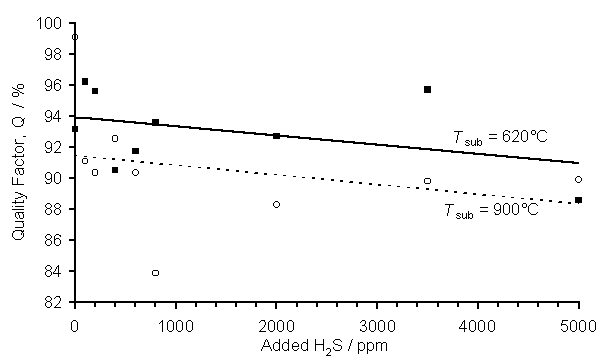
Figure 7.4. Plot of the
diamond film quality factor, Q, vs. H2S addition. Key: (■) Tsub = 620°C, (о) Tsub = 900°C, with other process
conditions as for Fig.7.1. The lines are
linear least squares fits to the two data sets (as labelled).
7.2.4. Four-point probe Film Resistivity Measurements
There is a general fall in resistivities with increased H2S addition, for the films grown at both high and low Tsub (Figure 7.4). The scatter seen for samples grown with low H2S additions and at high Tsub is due to the measurements being made at the limit of the apparatus capabilities. There is also a large offset of ~3 orders of magnitude between resistivity measurements of high and low Tsub grown films. Apart from this offset, the trend in film resistivities for H2S additions over 1000 ppm is similar for both datasets. Both show a ~100-fold reduction in resistivity when H2S additions are increased from 1000 to 5000 ppm.
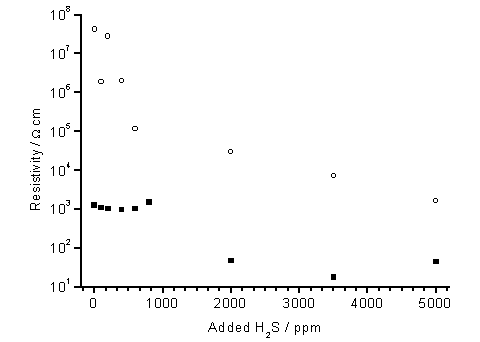
Figure 7.5. Plot of four
point probe resistivity vs. H2S addition for films deposited using H2S/51%CH4/49%CO2
gas mixtures. Key: (■) Tsub = 620°C, (о) Tsub = 900°C, with other process
conditions as for Fig.7.1.
7.2.5. XPS measurements of Film S/C ratio
XPS analysis has afforded S/C ratios of high temperature films grown with input gas mixture H2S concentrations of 3500 ppm (S/C ~0.07%) and 5000 ppm (S/C ~0.08%). We note that XPS yields an average signal over the whole area of the substrate (1 cm2), and so the position of the S (i.e. grain boundaries versus crystallites) cannot be determined. S/C ratios for all other deposited films were below the detection limit of the apparatus (~0.05%).
7.3. OES of H2S/51%CH4/49%CO2 Microwave Plasmas
An optical emission spectrum of a 1%H2S/51%CH4/49%CO2 MW plasma is presented in Figure 7.6. Emissions due to C2 Swan bands are clearly present at ~470, 516 and 560 nm. Also evident are emissions of the 3rd Positive and 5B bands (260-370 nm) and Angstrom bands (~451, 484, 520 and 561 nm) of CO. Emissions due to CH (431 nm) and H (483 nm) are also seen.
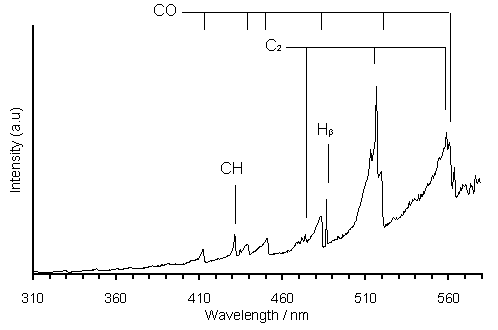
Figure 7.6. Plot of an
optical emission spectrum of a 1%H2S/51%CH4/49%CO2
MW plasma. Conditions: applied
microwave power 1 kW, pressure 40 Torr
Emission spectra taken of plasmas containing 0% and 1%H2S were almost identical. Therefore, six emission wavelengths were chosen and their relative intensities were plotted vs. H2S addition, as presented in Figure 7.7. It is clear that the intensities of the CO and H emission features stay relatively constant or even increase slightly over the range of H2S additions, while those of C2 and CH are seen to reduce by ~18 and 20%, respectively, over the same range. This suggests that increased H2S addition to the 51%CH4/49%CO2 gas mixture causes a reduction in hydrocarbon concentrations.

Figure 7.7. Optical emission
peak intensity vs. H2S addition to a 51%CH4/49%CO2
gas mixture. Emission features are as
labelled and percentages given indicate the drop in intensity of the indicated
feature over the range of H2S addition shown. Conditions: applied microwave power
1 kW, pressure 40 Torr.
7.4. Computer Simulations of H2S/51%CH4/49%CO2 Gas Mixtures
SENKIN computer simulations of the gas phase chemistry of H2S/51%CH4/49%CO2 gas mixtures were carried out using a combination of the GRI-mech 3.0, C-S linking and Leeds combined methane combustion mechanisms (see Sections 4.5.1 to 3). Figure 7.8 shows SENKIN predicted mole fractions, X, versus H2S addition to a 51%CH4/49%CO2 gas mixture at (a) 20 and (b) 40 Torr.
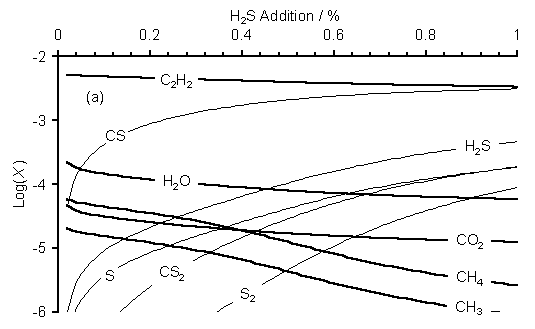
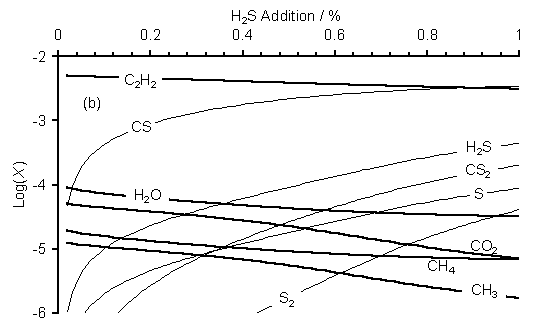
Figure 7.8. Plots of SENKIN
predicted species mole fraction, X, versus H2S addition to a
51%CH4/49%CO2 gas mixture for a pressure of (a) 20 and
(b) 40 Torr. Data for species not
containing S are presented as thick lines to aid clarity. Conditions:
simulation temperature 2000 K, reaction time 5 s.
It is clear from Figure 7.8 that the species trends are similar for the two pressures presented. In both cases H2S addition leads to a rise in predicted mole fraction of CS, [CS]. A rise is also seen in [H2S], [S], [CS2] and [S2], while [C2H2], [H2O], [CO2], [CH4] and [CH3] all fall with increased H2S addition. At both pressures [SO] and [SO2] are predicted to be ~10-7 and 10-11, respectively. Both species mole fractions are below the estimated detection limit of the MBMS apparatus of ~10-6. [H2] and [CO] are predicted to be 0.5 for both pressures. Increasing the simulation pressure from 20 to 40 Torr has the effect of decreasing the absolute values of [S2] while causing an increase in [CS2].
7.5. Molecular Beam Mass Spectrometry Studies of H2S/51%CH4/49%CO2 Microwave Plasmas
All MBMS measurements were made under the same conditions as the deposition runs, except that the chamber pressure was reduced from 40 to 20 Torr. This was because the presence of the probe made the plasma unstable at pressures >20 Torr. Measurements were made of H2S/51%CH4/49%CO2 MW activated gas mixtures as a function of H2S addition (0‑1%).
Figure 7.9 shows combined plots of MBMS measured species counts and SENKIN predicted species mole fractions, against added H2S. The respective vertical scales in each plot have been arranged so as to aid comparison. The plots of C2H2, CH4 and CH3 show good agreement between the measured and predicted species trends, with H2S addition resulting in a decrease in species mole fraction and measured counts. Similarly, experiment and simulation are in agreement in the case of H2S, CS and CS2, with species mole fraction and measured counts increasing upon increased H2S addition. There is, again, good agreement between MBMS and SENKIN results in the case of SO. However, the predicted mole fraction, X, of SO is ~10-7, which is approximately 100 times below the estimated detection limit of the MBMS apparatus. This suggests that SO is present within the microwave plasma at concentrations much greater than predicted by SENKIN. A similar conclusion can be drawn from the SENKIN and MBMS results for SO2. Here, the scatter in SO2 measured species counts data suggests SO2 concentrations are near the MBMS detection limit (X ~10-5), whereas the predicted SO2 mole fractions are far below this, i.e. ~10‑11. On the other hand, the MBMS measured counts of S and S2 appear to be at or below the MBMS detection limit, suggesting that the predicted mole fractions of S (~10‑4) and S2 (~10‑5) may be overestimates.

Figure 7.9. Plots of MBMS
measured species counts (right hand scale) and SENKIN predicted species mole
fractions (left hand scale) vs. H2S addition to a 51%CH4/49%CO2
gas mixture, for the following species: (i) C2H2, (ii) CH4,
(iii) CH3, (iv) H2S, (v) CS, (vi) CS2, (vii)
SO, (viii) SO2, (ix) S and (x) S2. Conditions are as given in Fig.7.1, except
that the pressure was reduced to 20 Torr to improve plasma stability in
the presence of the sampling probe.
SENKIN computer simulation results are for a H2S/51%CH4/49%CO2
gas mixture at a temperature of 2000 K and a pressure of
20 Torr. Key: о MBMS species counts, (·) SENKIN computer simulation
mole fractions. The two data sets in
each panel have been vertically scaled to illustrate the degree of consistency
between the experimental and modelled trends.
7.6. Discussion
The results presented in the preceding sections will be discussed and compared with those obtained using H2S/1%CH4/H2 gas mixtures (Chapter 6) in the following section. H2S addition to 51%CH4/49%CO2 MW plasmas during diamond CVD is seen to have a considerable impact upon the growth rates, quality and resistivities of the resulting films. S addition also induces changes in film morphology, particularly in the case of low temperature deposition, e.g. Tsub = 620°C. Reduction of Tsub from 900 to 620°C results in a ~10-fold drop in film growth rate. Such reduction in growth rates upon lowering of Tsub are consistent with previous observations (see Section 5.2.1 and Refs. [4] and [5]) but the increased substrate-plasma separation used during low Tsub deposition in the present studies may also contribute to the observed variation. However, the fact that we observe very similar downward trends in film growth rate for increased H2S additions at both deposition temperatures suggests that the trend is primarily due to gas phase reaction instigated by H2S, rather than gas-surface interactions.
A large offset was observed between the measured film resistivities of samples grown at high (900°C) and low (620°C) Tsub. We suggest that the lower resistivity of the low temperature grown samples is probably due to H-induced surface conduction [[6]-[9]]. This effect is reported to be annealed out of films at temperatures above ~700°C in vacuum and ~190°C in air [8]. The resistivity of such annealed films is several orders of magnitude greater than the H-terminated samples, pre-annealing. We therefore suspect that the growing diamond surface is (at least partially) H‑terminated at Tsub = 900°C. However, at the end of a deposition run the plasma is shut off and the film is left to cool down in a 100%CO2 atmosphere. During cooling from 900-700°C, we envisage that the H‑induced surface conductivity is annealed out, thereby resulting in the highly resistive film observed. Tsub = 620°C is below the temperature for such annealing. In this scenario, therefore, films grown at this lower substrate temperature would retain their H‑induced surface conductivity intact when cooled in a CO2 atmosphere. Previously films have been deposited at Tsub = 900°C using H2S/1%CH4/H2 gas mixtures (see Chapter 6). After growth, these films were cooled in a H2 atmosphere and the measured film resistivities (Section 6.2.4) were comparable with those obtained for the low temperature deposited films in the present work. This observation lends further credence to the hypothesis outlined above.
Increased H2S additions (>1000 ppm) appear to cause a reduction in film resistivities, regardless of Tsub. It is important to note that the reduction in film resistivities observed between high Tsub grown films with 0 and 5000 ppm H2S additions is much greater than the ~3-fold reduction seen for H2S doping of 1%CH4/H2 gas mixtures, using similar process conditions (Section 6.2.4). However, it remains unclear whether this reduction is a result of S-incorporation into the diamond films or due to an increase in graphitic phases within the film (as indicated by a parallel reduction in the quality factor).
OES measurements of C2 and CH emission features suggest that increased H2S additions caused a drop in gas phase hydrocarbon concentrations. This conclusion is reinforced by the observed trends in MBMS measured counts and SENKIN predicted mole fractions for C2H2, CH4, and CH3. The methyl radical is generally believed to be the major diamond growth precursor in most low pressure CVD reactors [[10],[11]]. It therefore seems reasonable to associate the measured drop in CH3 MBMS counts with the observed drop in film growth rates upon increased H2S addition.
A reaction scheme for the chemistry of H2S addition to 51%CH4/49%CO2 gas mixtures is presented in Table 7.1. It should be noted that all except Reaction 4 are composite conversions composed of many elementary reactions. Reaction 1 is the dominant reaction involving S containing species. This is shown by the large SENKIN predicted mole fractions and MBMS measured counts of CS2 and CS. Investigations of 50%CH4/50%CO2 gas mixtures (Section 5.7.3) have concluded that the majority of the input carbon is transformed to CO via Reaction 2. Any excess CH4 not destroyed by this reaction is able to undergo further conversions to produce C2H2 and CH3 via Reactions 3 and 4, respectively. However, Reaction 1 is able to compete with these reactions, with the result that increased H2S additions lead to a reduction in the CH4 available for production of C2H2, CH3 and H2O. This is analogous to the situation seen for H2S addition to 1%CH4/H2 gas mixtures (Section 6.8). As stated above, the reduction in CH3 concentrations caused by increased H2S addition leads to a reduction in deposited film growth rates.
|
|
Reaction |
DGreac (2000 K) / kJ mol-1 |
Keq (2000 K) |
|
1 |
CH4 + 2H2S Ë CS2 + 4H2 a) |
-172.1 |
3.124´104 |
|
2 |
CO2 + CH4Ë 2CO + 2H2 b) |
-306.0 |
1.014´108 |
|
3 |
2CH4 Ë C2H2 + 2H2 b) |
-144.4 |
5.916´103 |
|
4 |
CH4 + H Ë CH3 + H2 |
-53.30 |
24.67 |
|
5 |
CO2 + H2 Ë CO + H2O b) |
-25.00 |
5.477 |
|
6 |
CO2 + H2S Ë CO + H2 + SO |
32.45 |
1.420´10-1 |
|
7 |
H2O + H2S Ë SO + 2H2 |
60.74 |
2.592´10-2 |
|
8 |
CO2 + SO Ë CO + SO2 |
-36.34 |
8.893 |
a) Reaction taken from Section 5.7.3.
b) Reaction taken from Table 6.1 in Section 6.8.
Table 7.1. Proposed reaction
scheme for the gas phase chemistry of H2S/51%CH4/49%CO2
diamond CVD gas mixtures at 2000 K and ~20 Torr. DGreac values were calculated
using species Gibbs free energies of formation obtained from Reference [12]
and were also used to calculate reaction equilibrium constants, Keq.
In addition, we recall that there is a large (~2 mm) separation between the visible edge of the plasma and the substrate in the case of films grown at low Tsub, whereas the growth surface of samples deposited at high Tsub are in direct contact with the visible edge of the plasma. The larger substrate-plasma separation for low Tsub grown films clearly causes a further reduction in the CH3 flux reaching the substrate surface, as a result of radical recombination reactions (e.g. involving CH3 and H radicals). Arguably of even greater importance, is the reduced mobility of species across the growing film surface at low Tsub [5,[13],[14]]. Such diffusion is considered necessary for the growth of uniform diamond CVD films, as carbon containing species move to step edges allowing ordered crystal growth. These two effects are likely to be responsible for the much greater change in deposited film surface morphology observed upon H2S addition at lowered Tsub, compared to growth at the higher Tsub.
Table 7.1 also sets out two possible routes to the production of SO (Reactions 6 and 7). The first involves a composite reaction between CO2 and H2S producing CO, H2 and SO, while in the second reaction H2O and H2S react to form SO and hydrogen. At 2000 K, the Gibbs free energy for both reactions, DGreac, is greater than zero, indicating that, at equilibrium, the reverse of the reactions as written are spontaneous (i.e. DGreac < 0).
However, as stated previously, the MBMS measurements suggest that the SENKIN calculations underestimate the true SO and SO2 concentrations. This might reflect an overestimation of the forward rate constant of one or more of the elementary reactions of which Reaction 1 is comprised (Table 4.2 in Section 4.5.2), which would result in a reduced concentration of H2S available to participate in Reactions 6 and 7, and thus a lower predicted SO mole fraction. This, in turn, would cause an underestimation of the predicted mole fraction of SO2 produced via Reaction 8. Alternatively, or additionally, it may reflect inadequacies in the kinetic data for S/O and H containing species reactions that we have taken from the Leeds combined methane and SOx combustion mechanism (as discussed in Section 4.5.3). The fact that some 70% of these rate constants are either estimations or extrapolations from lower temperatures [[15]] serves to underline the inadequacy of the database of reactions involving H/C/O/S containing species.
Table 7.2 presents a comparison between SENKIN species mole fractions for 1%H2S addition to both 1%CH4/H2 and 51%CH4/49%CO2 gas mixtures. [H2], [C2H2], [H2S], and [CS] are predicted to be similar for both gas mixtures, whereas [CH3] and [CH4] are ~100 times smaller for the 1%H2S/51%CH4/49%CO2 gas mixture. For a 1%H2S/1%CH4/H2 gas mixture, [S] and [S2] are predicted to be at, or below, the estimated MBMS detection limit (in accordance with the lack of experimental measurements of these species). Changing to a 1%H2S/51%CH4/49%CO2 gas mixture has the effect of increasing [S] (~100 fold) and [S2] (~10 fold). This has the effect of increasing [S] to a level above the MBMS detection limit, as reflected by the experimental detection of S.
|
Species |
1%H2S/1%CH4/H2 (T = 1630 K) |
1%H2S/51%CH4/49%CO2 (T = 2000 K) |
|
CO |
|
4.84´10-1 |
|
CO2 |
|
1.24´10-5 |
|
H2O |
|
5.81´10-5 |
|
SO |
|
1.26´10-7 |
|
SO2 |
|
2.87´10-11 |
|
H2 |
9.89´10-1 |
5.01´10-1 |
|
CH4 |
1.71´10-3 |
2.58´10-6 |
|
CH3 |
1.95´10-5 |
8.91´10-7 |
|
C2H2 |
7.37´10-4 |
3.31´10-3 |
|
H2S |
1.12´10-3 |
4.74´10-4 |
|
S |
3.48´10-6 |
1.88´10-4 |
|
S2 |
1.17´10-5 |
9.36´10-5 |
|
CS |
4.46´10-3 |
3.12´10-3 |
|
CS2 |
1.97´10-3 |
1.97´10-3 |
Table 7.2. SENKIN predicted
species mole fractions for a 1%H2S addition to 1%CH4/H2
and 51%CH4/49%CO2 gas mixtures. Conditions: reaction time 5 s, Pressure 20 Torr,
temperature as shown for each gas mixture.
The S incorporation parameter, Fs = [CH3]´[CS], is plotted in Figure 7.10 as a function of H2S addition to both 1%CH4/H2 and 51%CH4/49%CO2 gas mixtures. It should be noted that the SENKIN product for the 51%CH4/49%CO2 gas mixture has been scaled vertically by a factor of five to fit onto the plot. XPS measured S/C ratios for films deposited using these gas mixtures (at Tsub = 900°C) are indicated also. The figure illustrates the point that, for a given H2S addition, 1%CH4/H2 gas mixtures are found to yield films with higher S/C ratios than 51%CH4/49%CO2 mixtures. Such an observation is consistent with the smaller SENKIN predicted values of [CH3]´[CS] calculated for the latter gas mixture. Indeed, this parameter is seen to maximise in the case of the H2S/51%CH4/49%CO2 gas mixtures, at ~0.42% added H2S. This is midway between the only two H2S addition values (0.35 and 0.5%) at which the S content was measurable by XPS.
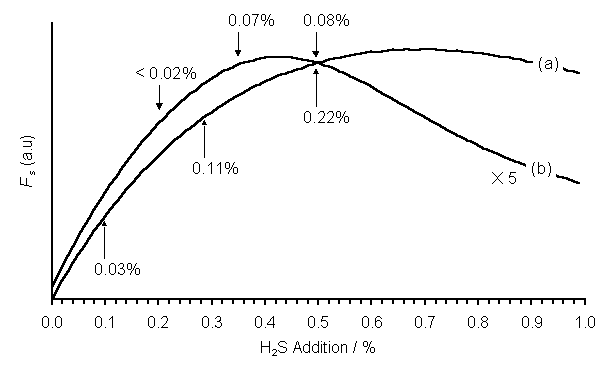
Figure 7.10. Plots of Fs
vs. H2S addition to (a) 1%CH4/H2 (Tgas = 1630 K)
and (b) 51%CH4/49%CO2 (Tgas = 2000 K)
gas mixtures obtained from the SENKIN calculations. Conditions: pressure 40 Torr, reaction time 5 s. The numbers present the S/C ratio (as
measured by XPS) for films deposited at the H2S additions
indicated. Note that the values of Fs
for the H2S/51%CH4/49%CO2 gas mixture have
been scaled up by a factor of 5.