Appendices
Appendix 1 : Resonance-Enhanced Multiphoton Ionisation (REMPI) Spectroscopy
Multiphoton ionisation (MPI) techniques rely on a species absorbing a sufficient number of photons to exceed its ionisation energy, thereby forming an ion and an electron. A biased probe wire or plate then detects either the ion or electron. The probability that the ionisation process occurs is greatly enhanced if the absorption of an integer number of photons is resonant with a real excited electronic state of the neutral species (typically a Rydberg state). This process allows the number of ions (or electrons) collected by a biased probe to be counted as a function of laser wavelength in order to produce a muliphoton ionisation spectrum.
REMPI schemes are typically written in the form (n + m) REMPI, where n signifies the number of photons used to promote the species into an eigenstate and m the number of further photons required to ionise the excited species, e.g. 2 +1 REMPI. This technique is extremely species selective as a significant ionisation signal is only detected when the incident laser wavelength is tuned so that the process is resonance enhanced.
MPI also has the advantage that the selection rules for multiphoton excitation permit promotion into levels that are not normally accessible by one-photon excitation from the ground state. The range of tunability of the laser is also multiplied by the number of photons required for the excitation of the Rydberg level, significantly broadening the spectral region that can be probed with a given laser.
REMPI provides a method of detecting non-fluorescing radicals such as CH3, thought to be an important precursor for diamond growth, and therefore is a valuable diagnostic technique.
In the context of diamond CVD, REMPI has been previously used to observe H atoms and CH3 radicals in a hot filament reactor. However, the abundance of free electrons in microwave and DC-arcjet reactors prevent its use.
Appendix 2 : Laser
Induced Fluorescence (LIF) Spectroscopy
LIF techniques operate by detection of fluorescence from a species observed after excitation with a laser pulse. The laser pulse, at a wavelength to coincide with absorption of the species, promotes the species into an excited state. The consequent rapid fluorescent decay is detected with a photomultiplier tube, typically positioned at right angles to the incident laser beam.
Both the excitation and fluorescence wavelengths are species selective and the fluorescence signal is proportional to the population density in the excited state, and thus, in the absence of saturation effects, to the population in the initial state excited with the laser.
Tuning the laser wavelength therefore produces an excitation spectrum of the species of interest. LIF is an unobtrusive, highly species selective technique that, with careful calibration, is capable of yielding absolute species number densities and is therefore considered to be ideal for monitoring species present in the highly volatile gas-phase environment typical in diamond CVD. LIF techniques have previously been used to monitor CH, C2 and C2H in DC-arcjet reactors.
However, LIF techniques cannot detect non-fluorescing radicals. The methyl radical, CH3, cannot be detected by LIF due to the predissociative nature of its upper state, and has been detected via REMPI and CRDS. It is also difficult to use in highly luminous environments, such as DC-arcjets.
Appendix 3 : Cavity
Ringdown Spectroscopy (CRDS)
CRDS is, in essence, a highly sensitive absorption technique. The increased sensitivity over standard absorption techniques comes from augmenting the number of times the light passes through the absorbing medium, and measuring the change in the rate of light intensity decay as a function of time. In a traditional absorption experiment, depletion of the exciting light intensity is monitored.
Typical CRDS experiments use a pulsed tunable laser to pass light into a cavity, defined by two highly reflective (>99.5%) mirrors, and measure the decay of reflected light escaping from the exit mirror with a photomultiplier tube (PMT). If the cavity contains a species that absorbs the radiation produced by the laser, the rate of decay will be faster than that of an empty (or non-absorbing) cavity. The change in the decay trace coefficients may be used to calculate the absorption coefficient which, given the absorption cross section of the species of interest, may be used to obtain absolute column densities. To obtain local concentrations one, of course, also needs an idea of the density profile along the column that is being probed.
In terms of diamond CVD, CRDS has previously been used successfully to monitor CH3 radical number densities in a hot filament reactor. As CRDS is capable of yielding absolute species number densities in a hostile environment, increased interest has recently been shown in this technique in the study of species in microwave or, as here, arcjet reactors.
Appendix 4 : Optical
emission spectroscopy (OES)
OES is used in the study of highly excited species that emit radiation in the wavelength range from the near UV to the near IR. The high gas temperatures induce the spontaneous emission observed in DC-arcjet and microwave CVD reactors. In such reactors the emission observed from H2/CH4 gas mixtures is mainly due to the C2 Swan band system, the H-Balmer series and the A-X transition of CH.
This underlines the main limitation with OES measurements; that very few species transitions are detectable in the wavelength range that may be monitored conveniently. This applies even to simple diatomic species like H2 and 1C2. Monitoring IR emission from vibrationally excited ground state species is another possibility, but quenching under typical process conditions is a problem, as is the spectral complexity given the high gas temperatures involved.
It is also relevant to note diamond growth is fundamentally achieved by species in their ground state. OES, however, detects the decay from excited states. Information from OES is thus relevant to diamond growth only if the correlation between ground and excited state species is established. This said, OES has previously been used extensively in the detection of species in the diamond depositing gas-phase environment in microwave, DC-arcjet and hot filament reactors, not least for process control purposes.
Typically, emission from the gas-phase is passed through a monochromator. Spectra are obtained either by rotating a diffraction grating and monitoring just the fraction of emission passing through the exit slit, or by collecting all the emission dispersed from the grating on a strip CCD detector.
OES has also proven useful in the determination of gas-phase temperatures in the CVD environment, with the rotational temperatures of C2 and CH being calculated from the collected OES spectrum, assuming a Boltzmann population distribution over the various emitting rovibrational states.
Appendix 5 : Saha
Equation
The Saha equation describes the degree of
different stages of ionization of a species under the assumption of Local
Thermodynamic Equilibrium (LTE).
The equation allows for the fact that while the
environment, theoretically, prevents the occurrence of species ionisation, the
total number of free states is such that ionisation is promoted.
If we consider a system whereby the Boltzmann
equation may be used to calculate the number density of ground state ionized
atoms (n*), relative to ground state neutral atoms (n) then,
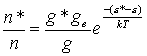
![]() Equation
A.1
Equation
A.1
where g* and g are the respective degeneracies of
the ionised and neutral atoms, k is the Boltzmann constant, and the term (e*-e) describes the difference in energy
between the atom and the ion. The
degeneracy (or statistical weight) of the free-electron state may be quoted as,
![]() Equation
A.2
Equation
A.2
where m is the electron mass, ne is the electron number density, u is the velocity of the electron and
h is
Planck’s constant. Given that in equation
A.1 the difference of energy between the atom and ion includes the ionization
energy and the electron energy then,
![]() Equation
A.3
Equation
A.3
where c is the ionization energy. By combining equations A.1, A.2 and A.3 and
integrating over all electron velocities the general Saha equation may be
written as,
![]() . Equation
A.4
. Equation
A.4
The Saha equation allows the state of the
ionisation processes in a system to be calculated. Examination of the equation also predicts a number of interesting
features. Firstly, the degree of
ionisation in the system will be greater than that predicted by calculations
based on the local gas temperature and the species ionisation potential. Secondly, since, in general, the ground
state of the ion lies well below the various excited states, the ions produced
are predominantly in their ground state.
However, the Saha equation assumes LTE, which
is considered unlikely in the diamond CVD environment, this therefore causes
the limitation that it is strictly only applicable if elastic collisions are
responsible for establishing the energetic distribution of particles. In most practical cases radiative processes
will be more important, and an explicit detailed equilibrium calculation is
necessary in order to determine the distribution of electrons over the various
energy-levels.
Appendix 6 : GRI_MECH 3.0 (C / H / N) Gas-phase
chemistry
This table shows all reactions used in the numerical simulations detailed in the modelling of a HF-CVD reactor operating on a CH4/NH3/H2 gas mixture, with all irrelevant reactions removed, i.e. those involving oxygen or argon. The reaction rate co-efficients shown are such that the reaction rate constant, k, may be calculated from,
![]() . Equation
A.5
. Equation
A.5
The coefficients listed here have the units; A (cm3 mol-1s-1), b is dimensionless and Ea (cal mol-1K-1). The entire mechanism is shown here, where M signifies a third body. For such three body reactions, the following three lines detail the relative third body efficiencies. The prefix LOW or TROE designates a set of constants that describe respectively, the falloff rates for low-pressure rate limits, and the falloff form to describe pressure effects as presented by J. Troe†.
Reaction A b Ea
2H+M<=>H2+M 1.000E+18 -1.000 .00
H2/
.00/ CH4/2.00/ C2H6/3.00/
2H+H2<=>2H2 9.000E+16 -.600 .00
H+CH<=>C+H2 1.650E+14 .000 .00
H+CH2(+M)<=>CH3(+M) 6.000E+14 .000 .00
LOW
/ 1.040E+26 -2.760
1600.00/
TROE/
.5620 91.00 5836.00
8552.00/
H2/2.00/
CH4/2.00/ C2H6/3.00/
H+CH2(S)<=>CH+H2 3.000E+13 .000 .00
H+CH3(+M)<=>CH4(+M) 13.90E+15 -.534
536.00
LOW
/ 2.620E+33 -4.760
2440.00/
TROE/
.7830 74.00 2941.00
6964.00 /
H2/2.00/
CH4/3.00/ C2H6/3.00/
H+CH4<=>CH3+H2 6.600E+08 1.620
10840.00
H+C2H(+M)<=>C2H2(+M) 1.000E+17 -1.000 .00
LOW
/ 3.750E+33 -4.800
1900.00/
TROE/
.6464 132.00 1315.00
5566.00 /
H2/2.00/
CH4/2.00/ C2H6/3.00/
H+C2H2(+M)<=>C2H3(+M) 5.600E+12 .000
2400.00
LOW
/ 3.800E+40 -7.270
7220.00/
TROE/
.7507 98.50 1302.00
4167.00 /
H2/2.00/
CH4/2.00/ C2H6/3.00/
H+C2H3(+M)<=>C2H4(+M) 6.080E+12 .270
280.00
LOW
/ 1.400E+30 -3.860
3320.00/
TROE/
.7820 207.50 2663.00
6095.00 /
H2/2.00/
CH4/2.00/ C2H6/3.00/
H+C2H3<=>H2+C2H2 3.000E+13 .000 .00
H+C2H4(+M)<=>C2H5(+M) 0.540E+12 .454
1820.00
LOW
/ 0.600E+42 -7.620
6970.00/
TROE/
.9753 210.00 984.00
4374.00 /
H2/2.00/
CH4/2.00/ C2H6/3.00/
H+C2H4<=>C2H3+H2 1.325E+06 2.530
12240.00
H+C2H5(+M)<=>C2H6(+M) 5.210E+17 -.990
1580.00
LOW
/ 1.990E+41 -7.080
6685.00/
TROE/
.8422 125.00 2219.00
6882.00 /
H2/2.00/
CH4/2.00/ C2H6/3.00/
H+C2H5<=>H2+C2H4 2.000E+12 .000 .00
H+C2H6<=>C2H5+H2 1.150E+08 1.900
7530.00
C+CH2<=>H+C2H 5.000E+13 .000 .00
C+CH3<=>H+C2H2 5.000E+13 .000 .00
CH+H2<=>H+CH2 1.080E+14 .000
3110.00
CH+CH2<=>H+C2H2 4.000E+13 .000
.00
CH+CH3<=>H+C2H3 3.000E+13 .000 .00
CH+CH4<=>H+C2H4 6.000E+13 .000 .00
CH2+H2<=>H+CH3 5.000E+05 2.000
7230.00
2CH2<=>H2+C2H2 1.600E+15
.000 11944.00
CH2+CH3<=>H+C2H4 4.000E+13 .000 .00
CH2+CH4<=>2CH3 2.460E+06 2.000
8270.00
CH2(S)+H2<=>CH3+H 7.000E+13 .000 .00
CH2(S)+CH3<=>H+C2H4 1.200E+13 .000
-570.00
CH2(S)+CH4<=>2CH3 1.600E+13 .000
-570.00
CH2(S)+C2H6<=>CH3+C2H5 4.000E+13 .000
-550.00
2CH3(+M)<=>C2H6(+M) 6.770E+16 -1.180
654.00
LOW
/ 3.400E+41 -7.030
2762.00/
TROE/
.6190 73.20 1180.00
9999.00 /
H2/2.00/
CH4/2.00/ C2H6/3.00/
2CH3<=>H+C2H5 6.840E+12 .100
10600.00
CH3+C2H4<=>C2H3+CH4 2.270E+05 2.000
9200.00
CH3+C2H6<=>C2H5+CH4 6.140E+06 1.740
10450.00
C2H+H2<=>H+C2H2 5.680E+10 0.900
1993.00
C2H4(+M)<=>H2+C2H2(+M) 8.000E+12 .440
86770.00
LOW
/ 1.580E+51 -9.300
97800.00/
TROE/
.7345 180.00 1035.00
5417.00 /
H2/2.00/
CH4/2.00/ C2H6/3.00/
CH+H2(+M)<=>CH3(+M) 1.970E+12 .430
-370.00
LOW/ 4.820E+25 -2.80 590.0 /
TROE/ .578
122.0 2535.0 9365.0 /
H2/2.00/
CH4/2.00/ C2H6/3.00/
CH2+CH2=>2H+C2H2 2.000E+14 .000
10989.00
CH3+C2H5(+M)<=>C3H8(+M) .9430E+13 .000 .00
LOW/ 2.710E+74 -16.82 13065.0 /
TROE/ .1527 291.0 2742.0 7748.0 /
H2/2.00/
CH4/2.00/ C2H6/3.00/
H+C3H8<=>C3H7+H2 1.320E+06 2.540
6756.00
CH3+C3H8<=>C3H7+CH4 0.903E+00 3.650
7154.00
CH3+C2H4(+M)<=>C3H7(+M) 2.550E+06 1.600
5700.00
LOW/ 3.00E+63 -14.6 18170./
TROE/ .1894 277.0 8748.0 7891.0 /
H2/2.00/
CH4/2.00/ C2H6/3.00/
H+C3H7(+M)<=>C3H8(+M) 3.613E+13 .000 .00
LOW/ 4.420E+61 -13.545 11357.0/
TROE/ .315 369.0 3285.0 6667.0 /
H2/2.00/
CH4/2.00/ C2H6/3.00/
H+C3H7<=>CH3+C2H5 4.060E+06 2.190
890.00
CH3+C3H7<=>2C2H5 1.927E+13 -0.320 .00
NH+H<=>N+H2 3.200E+13 .000
330.00
NH+N<=>N2+H 1.500E+13
.000 .00
NH2+H<=>NH+H2 4.000E+13 .000
3650.00
NNH<=>N2+H 3.300E+08 .000 .00
NNH+M<=>N2+H+M 1.300E+14 -.110
4980.00
H2/2.00/
CH4/2.00/ C2H6/3.00/
NNH+H<=>H2+N2 5.000E+13 .000 .00
NNH+CH3<=>CH4+N2 2.500E+13 .000 .00
CN+H2<=>HCN+H 2.950E+05 2.450
2240.00
HCN+M<=>H+CN+M 1.040E+29 -3.300
26600.00
H2/2.00/
CH4/2.00/ C2H6/3.00/
H+HCN(+M)<=>H2CN(+M) 3.300E+13 .000 .00
LOW /
1.400E+26 -3.400 1900.00/
H2/2.00/
CH4/2.00/ C2H6/3.00/
H2CN+N<=>N2+CH2 6.000E+13
.000 400.00
C+N2<=>CN+N 6.300E+13 .000
46020.00
CH+N2<=>HCN+N 3.120E+09 0.880
20130.00
CH+N2(+M)<=>HCNN(+M) 3.100E+12 .150
.00
LOW
/ 1.300E+25 -3.160
740.00/
TROE/
.6670 235.00 2117.00
4536.00 /
H2/2.00/
CH4/2.00/ C2H6/3.00/
CH2+N2<=>HCN+NH 1.000E+13 .000
74000.00
CH2(S)+N2<=>NH+HCN 1.000E+11 .000
65000.00
HCNN+H<=>CH2+N2 1.000E+14 .000 .00
CH3+N<=>H2CN+H 6.100E+14 -.310
290.00
CH3+N<=>HCN+H2 3.700E+12 .150
-90.00
NH3+H<=>NH2+H2 5.400E+05
2.400 9915.00
Appendix 7 : Laser Raman Spectroscopy (LRS)
If monochromatic radiation is passed through a substance most of the scattered radiation will be of the incident frequency (Rayleigh Scattering). However, certain discrete frequencies above and below that of the incident radiation (Raman scattering) will also be present. Elastically and inelastically scattered photons are detected with energies of, hn and hn ± DE, where n is the frequency of the incident beam.
The inelastically scattered photons, having exchanged energy with the sample, will be of frequency, n ±DE/h, where DE coincides with a vibrational (or rotational) transition in the case of an isolated molecule, or a phonon mode in the case of a solid. If the sample gains energy the scattered photon will be of frequency, n -DE/h this is known as Stokes’ radiation. Conversely, if the sample loses energy the scattered photon is known as anti-Stokes’ radiation, (i.e. n +DE/h).
In the context of diamond analysis, LRS is an important technique as the vibrational modes within the bulk crystal are sensitive to the nature of the bonding. LRS is capable of showing both sp3 bonded carbon (sharp Raman peak centred at ~1333 cm-1) typical of diamond, and sp2 graphitic carbon (broad Raman peak centred around 1550 cm-1). Therefore LRS has become useful as a diagnostic tool for, as-grown CVD diamond films, providing information of the diamond/graphitic content.